Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.molliq.2020.113767 Anna Ignaczak , Łukasz Orszański , Marta Adamiak , Agnieszka B. Olejniczak
|
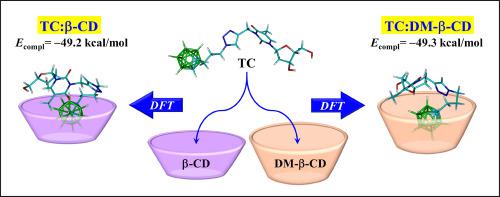
Carboranes are unusual compounds with unique structural and chemical properties, which play an increasingly important role in the design of new potential pharmaceuticals. One of the noteworthy properties of closo-carboranes is their very high hydrophobicity, which can facilitate transport of carborane-containing compounds across biological membranes, but at the same time may limit their therapeutic availability. This problem can be solved by complexing the drug with cyclodextrins. In the present work, properties of thymidine-carborane conjugate (TC) and its ability to form in aqueous solution inclusion complexes with two cyclodextrins: β-CD and DM-β-CD, are investigated using the density functional (DFT) method B3LYP-GD2/6-31G (d, p) and the Polarizable Continuum Model (PCM) of water. The most stable structures of the molecules alone TC, DM-β-CD, as well as of the complexes TC: β-CD and TC: DM-β-CD in water, are presented. It is shown that in aqueous solution TC preferentially adopts a twisted shape, which differs significantly from its crystallographic structure. In the lowest energy complexes with both cyclodextrins, TC is docked in the host molecule via the wider entry point of CD, with the carborane cluster completely submersed in the cyclodextrin cavity. The corresponding complexation energies for TC: β-CD and TC: DM-β-CD are very similar, of about −49 kcal/mol, but the formation of TC: DM-β-CD is thermodynamically more favored. The solvation, interaction and deformation energies, as well as frontier orbitals in the complexes are analyzed in detail. An effect of the complex formation on the IR and NMR spectra of the individual molecules is also presented and discussed.
中文翻译:

胸腺嘧啶-碳硼烷共轭物与β-环糊精和庚七(2,6- O-二甲基)-β-环糊精包合物的比较DFT研究
碳硼烷是具有独特结构和化学性质的不寻常化合物,在新的潜在药物设计中起着越来越重要的作用。Closo的值得注意的特性之一-碳硼烷具有很高的疏水性,可以促进含碳硼烷的化合物跨生物膜的运输,但同时可能会限制其治疗可用性。通过将药物与环糊精复合可以解决该问题。在本工作中,使用密度泛函(DFT)方法B3LYP-B研究了胸苷-碳氢化合物共轭物(TC)的性质及其在具有两种环糊精的水溶液包合物中形成的能力:β-CD和DM-β-CD。 GD2 / 6-31G(d,p)和水的可极化连续体模型(PCM)。给出了单独的分子TC,DM-β-CD以及复合物TC:β-CD和TC:DM-β-CD在水中的最稳定结构。结果表明,在水溶液中TC优先采用扭曲形状,其晶体结构明显不同。在两种环糊精的最低能量复合物中,TC通过更宽的CD进入点对接在宿主分子中,而碳硼烷簇完全浸没在环糊精腔中。TC:β-CD和TC:DM-β-CD的相应络合能非常相似,约为-49 kcal / mol,但热力学上更有利于TC:DM-β-CD的形成。详细分析了复合物中的溶剂化,相互作用和形变能以及前沿轨道。还介绍和讨论了复合物形成对单个分子的IR和NMR光谱的影响。碳硼烷簇完全浸没在环糊精腔中。TC:β-CD和TC:DM-β-CD的相应络合能非常相似,约为-49 kcal / mol,但热力学上更有利于TC:DM-β-CD的形成。详细分析了复合物中的溶剂化,相互作用和形变能以及前沿轨道。还介绍和讨论了复合物形成对单个分子的IR和NMR光谱的影响。碳硼烷簇完全浸没在环糊精腔中。TC:β-CD和TC:DM-β-CD的相应络合能非常相似,约为-49 kcal / mol,但热力学上更有利于TC:DM-β-CD的形成。详细分析了复合物中的溶剂化,相互作用和形变能以及前沿轨道。还介绍和讨论了复合物形成对单个分子的IR和NMR光谱的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号