European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.ejmech.2020.112529 Sylwia Sudoł 1 , Katarzyna Kucwaj-Brysz 2 , Rafał Kurczab 3 , Natalia Wilczyńska 4 , Magdalena Jastrzębska-Więsek 4 , Grzegorz Satała 3 , Gniewomir Latacz 1 , Monika Głuch-Lutwin 5 , Barbara Mordyl 5 , Ewa Żesławska 6 , Wojciech Nitek 7 , Anna Partyka 4 , Kamila Buzun 8 , Agata Doroz-Płonka 1 , Anna Wesołowska 4 , Anna Bielawska 9 , Jadwiga Handzlik 1
|
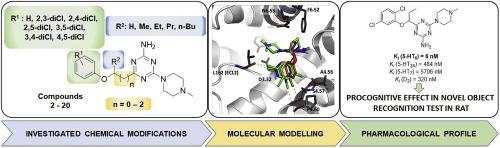
In the light of recent lines of evidence, 5-HT6R ligands are a promising tool for future treatment of memory impairment. Hence, this study has supplied highly potent 5-HT6R agents with procognitive effects, which represent an original chemical class of 1,3,5-triazines, different from widely studied sulfone and indole-like 5-HT6R ligands. The new compounds were rationally designed as modifications of lead, 4-(1-(2-chlorophenoxy)ethyl)-6-(4-methylpiperazin-1-yl)-1,3,5-triazin-2-amine (1), involving an introduction of: (i) two chlorines at benzene ring and (ii) varied linkers joining the triazine ring to aromatic ethers. Synthesis, in vitro and in vivo biological tests and computer-aided SAR analysis for 19 new compounds were carried out. Most of the new triazines displayed high affinity (Ki < 100 nM) and selectivity towards 5-HT6R, with respect to 5-HT2AR, 5-HT7R and D2R. The crystallography-supported docking studies, including quantum-polarized ligand docking (QPLD), indicated that chlorine atoms may be involved in different type of halogen bonding, however, the linker properties seem to predominately affect the 5-HT6R affinity. 4-[1-(2,5-Dichlorophenoxy)propyl]-6-(4-methylpiperazin-1-yl)-1,3,5-triazin-2-amine (9), which displayed: the highest affinity (Ki = 6 nM), very strong 5-HT6R antagonistic action (KB = 27 pM), procognitive effects in vivo in novel object recognition (NOR) test in rats, a very good permeability in PAMPA model and satisfying safety in vitro, was identified as the most potent 1,3,5-triazine agent so far, useful as a new lead for further research.
中文翻译:

氯取代基和接头拓扑结构是5-HT6R活性的因子,对体内具有认知功能的新型高活性1,3,5-三嗪衍生物具有重要意义。
根据最近的证据,5-HT 6 R配体是未来治疗记忆障碍的有前途的工具。因此,本研究提供了具有认知作用的强效5-HT 6 R药物,它代表了1,3,5-三嗪的原始化学类别,与广泛研究的砜和吲哚样5-HT 6 R配体不同。新化合物经过合理设计,是对铅4-(1-(2-氯苯氧基)乙基)-6-(4-甲基哌嗪-1-基)-1,3,5-三嗪-2-胺的修饰(1) ,涉及引入:(i)苯环上的两个氯和(ii)将三嗪环连接到芳族醚上的各种连接基。合成,体外和对19种新化合物进行了体内生物学测试和计算机辅助SAR分析。相对于5-HT 2A R,5-HT 7 R和D 2 R,大多数新的三嗪类 化合物对5-HT 6 R表现出高亲和力(K i <100 nM)和选择性。晶体学支持的对接研究包括量子极化的配体对接(QPLD),表明氯原子可能参与不同类型的卤素键,但是,连接子的性质似乎主要影响5-HT 6 R的亲和力。4- [1-(2,5-二氯苯氧基)丙基] -6-(4-甲基哌嗪-1-基)-1,3,5-三嗪-2-胺(9),显示:最高亲和力(Ki = 6 nM),非常强的5-HT 6 R拮抗作用( K B = 27 pM),在大鼠的新对象识别(NOR)测试中的体内认知作用,在PAMPA模型中具有很好的渗透性并满足体外安全性,被确定为迄今为止最有效的1,3,5-三嗪试剂,可用作进一步研究的新线索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号