Electrochimica Acta ( IF 6.6 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.electacta.2020.136700 Faezeh Zivari-Moshfegh , Davood Nematollahi , Mahmood Masoudi Khoram , Abdollah Rahimi
|
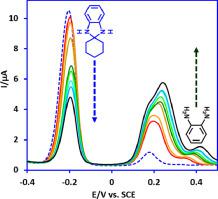
Electrochemical behavior of o-phenylenediamine (PDA) and 1,3-dihydrospiro[benzo[d]imidazole-2,1′-cyclohexane] (DBI) was extensively studied in water and water/ethanol mixture using different voltammetric techniques. Our data showed that the oxidation of PDA is highly dependent on pH, follows a complex pattern and participate in following chemical reactions such as polymerization. Unlike acidic and neutral solutions, in highly alkaline solutions (pH ≥ 11), however, PDA shows a simple reversible redox system. Contrary to PDA, the presence of the cyclohexyl group in the structure of DBI makes its oxidation pattern less complex than that of PDA and causes the molecule less susceptible to participate in the following chemical reactions. Our results showed that DBI in aqueous solutions is unstable and undergoes acid catalyzed hydrolysis to give PDA. The instability of DBI in acidic solutions is so high that it turns completely into PDA in the time scale of the voltammetric experiments. Different from acidic media, in alkaline solutions (pH ≥ 9.0), the hydrolysis rate is slow, so that DBI shows a reversible redox couple. The kinetic of DBI hydrolysis using differential pulse voltammetry method was studied and the apparent hydrolysis rate constants () were found by assuming the pseudo-first order rate kinetics. In addition, in this work, adsorption activity, diffusion coefficient and pKa values of DBI and PDA species were determined and the Pourbaix diagrams for these compounds were constructed. The most important part of this paper is devoted to introducing a rare type of mechanism in electrochemical reactions. In this way, the rarely studied mechanism has been introduced in relation to the reaction of PDA with cyclohexanone and the formation of DBI.
中文翻译:

邻苯二胺和1,3二氢螺[苯并[ d ]咪唑-2,1'-环己烷]的电化学氧化。全面研究并介绍CE机制的新案例
的电化学行为ø苯二胺(PDA)和1,3-二氢螺[苯并[ d使用不同的伏安技术在水和水/乙醇混合物中对[]咪唑-2,1'-环己烷](DBI)进行了广泛的研究。我们的数据表明PDA的氧化高度依赖于pH值,遵循复杂的模式并参与以下化学反应(例如聚合反应)。与酸性和中性溶液不同,在高碱性溶液(pH≥11)中,PDA显示出简单的可逆氧化还原系统。与PDA相反,DBI结构中环己基的存在使其氧化方式不如PDA复杂,并且使分子不易参与以下化学反应。我们的结果表明,水溶液中的DBI不稳定,并且经过酸催化水解生成PDA。DBI在酸性溶液中的不稳定性非常高,以至于在伏安法实验的时间范围内完全变成了PDA。与酸性介质不同,在碱性溶液(pH≥9.0)中,水解速度较慢,因此DBI显示出可逆的氧化还原对。研究了用微分脉冲伏安法研究DBI的水解动力学,并给出了表观水解速率常数(通过假设伪一级速率动力学发现。此外,在这项工作中,测定了DBI和PDA种类的吸附活性,扩散系数和p K a值,并绘制了这些化合物的Pourbaix图。本文最重要的部分致力于介绍电化学反应中一种罕见的机理。这样,关于PDA与环己酮的反应以及DBI的形成,已经引入了很少研究的机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号