Developmental Cell ( IF 11.8 ) Pub Date : 2020-07-07 , DOI: 10.1016/j.devcel.2020.06.017 Ignas Gaska 1 , Mason E Armstrong 1 , April Alfieri 1 , Scott Forth 1
|
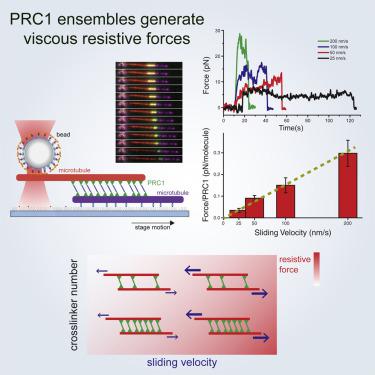
Cell division in eukaryotes requires the regulated assembly of the spindle apparatus. The proper organization of microtubules within the spindle is driven by motor proteins that exert forces to slide filaments, whereas non-motor proteins crosslink filaments into higher-order motifs, such as overlapping bundles. It is not clear how active and passive forces are integrated to produce regulated mechanical outputs within spindles. Here, we employ simultaneous optical trapping and total internal reflection fluorescence (TIRF) microscopy to directly measure the frictional forces produced by the mitotic crosslinking protein PRC1 that resist microtubule sliding. These forces scale with microtubule sliding velocity and the number of PRC1 crosslinks but do not depend on overlap length or PRC1 density within overlaps. Our results suggest that PRC1 ensembles act similarly to a mechanical dashpot, producing significant resistance against fast motions but minimal resistance against slow motions, allowing for the integration of diverse motor activities into a single mechanical outcome.
中文翻译:

有丝分裂交联蛋白PRC1的行为就像一个机械仪表盘,以抵抗微管滑动。
真核生物中的细胞分裂需要纺锤体设备的调节组装。纺锤体内微管的正确组织是由运动蛋白驱动的,该蛋白施加力使细丝滑动,而非运动蛋白将细丝交联成更高阶的基序,例如重叠的束。尚不清楚主动和被动力如何集成以在主轴内产生调节的机械输出。在这里,我们采用同时光学捕获和全内反射荧光(TIRF)显微镜直接测量由有丝分裂交联蛋白PRC1产生的抵抗微管滑动的摩擦力。这些力与微管滑动速度和PRC1交联的数量成比例,但不取决于重叠长度或重叠内的PRC1密度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号