当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Polymer chain editing: functionality “knock-in”, “knock-out” and replacement via cross metathesis reaction and thiol-Michael addition
Polymer Chemistry ( IF 4.6 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0py00549e Jie Ren 1, 2, 3, 4, 5 , Junpo He 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.6 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0py00549e Jie Ren 1, 2, 3, 4, 5 , Junpo He 1, 2, 3, 4, 5
Affiliation
|
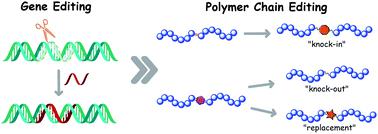
We hereby report a strategy for polymer modification including the operation of functionality “knock-in”, “knock-out” and replacement on the polymer main chains through the olefin cross metathesis (CM) reaction followed by thiol-Michael addition. The model polymers containing olefin moieties were prepared by atom transfer radical polymerization (ATRP) using designed dibromide initiators possessing double bond(s) and specific functionalities. The CM reaction between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the polymer chain and acrylates was of high efficiency, resulting in nearly 100% “scissored” chains which were subsequently recombined via thiol-Michael addition with different dithiol coupling agents. Compared with the initial ATRP products, the rebuilt polymers have almost the same molecular weight, molecular weight distribution, and chemical composition. Thus, functionality “knock-in” was fulfilled by CM between the model polymer and acrylate followed by recombination using the dithiol compound bearing the desired functionality. Functionality “knock-out” and replacement were accomplished by CM between the model polymer and acrylate followed by recombination using the dithiol compound without and with another functionality, respectively. Owing to the mild conditions and great tolerance of both olefin metathesis and thiol-Michael addition, the present strategy can serve as a useful tool for polymer post-editing through the unique process of chain cleavage (cross metathesis reaction) and recombination (thiol-Michael addition).
C bonds in the polymer chain and acrylates was of high efficiency, resulting in nearly 100% “scissored” chains which were subsequently recombined via thiol-Michael addition with different dithiol coupling agents. Compared with the initial ATRP products, the rebuilt polymers have almost the same molecular weight, molecular weight distribution, and chemical composition. Thus, functionality “knock-in” was fulfilled by CM between the model polymer and acrylate followed by recombination using the dithiol compound bearing the desired functionality. Functionality “knock-out” and replacement were accomplished by CM between the model polymer and acrylate followed by recombination using the dithiol compound without and with another functionality, respectively. Owing to the mild conditions and great tolerance of both olefin metathesis and thiol-Michael addition, the present strategy can serve as a useful tool for polymer post-editing through the unique process of chain cleavage (cross metathesis reaction) and recombination (thiol-Michael addition).
中文翻译:

聚合物链编辑:功能“敲入”,“敲除”以及通过交叉易位反应和硫醇-迈克尔加成的替换
我们在此报告聚合物改性的策略,包括功能性“敲入”,“敲除”和通过烯烃交叉复分解(CM)反应接着硫醇-迈克尔加成反应在聚合物主链上进行取代的操作。使用设计的具有双键和特定官能度的二溴化物引发剂,通过原子转移自由基聚合(ATRP),制备了包含烯烃部分的模型聚合物。![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 聚合物链中的C C键与丙烯酸酯之间的CM反应效率很高,产生了近100%的“剪刀”链,随后这些链通过用不同的二硫醇偶联剂添加硫醇-迈克尔。与最初的ATRP产品相比,重建的聚合物具有几乎相同的分子量,分子量分布和化学组成。因此,通过CM在模型聚合物和丙烯酸酯之间实现官能团“敲入”,然后使用带有所需官能团的二硫醇化合物进行重组。通过模型聚合物和丙烯酸酯之间的CM进行功能“敲除”和替换,然后分别使用不含和具有其他功能的二硫醇化合物进行重组。由于烯烃复分解反应和巯基-迈克尔加成反应的条件温和且耐受性强,
聚合物链中的C C键与丙烯酸酯之间的CM反应效率很高,产生了近100%的“剪刀”链,随后这些链通过用不同的二硫醇偶联剂添加硫醇-迈克尔。与最初的ATRP产品相比,重建的聚合物具有几乎相同的分子量,分子量分布和化学组成。因此,通过CM在模型聚合物和丙烯酸酯之间实现官能团“敲入”,然后使用带有所需官能团的二硫醇化合物进行重组。通过模型聚合物和丙烯酸酯之间的CM进行功能“敲除”和替换,然后分别使用不含和具有其他功能的二硫醇化合物进行重组。由于烯烃复分解反应和巯基-迈克尔加成反应的条件温和且耐受性强,
更新日期:2020-07-28
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the polymer chain and acrylates was of high efficiency, resulting in nearly 100% “scissored” chains which were subsequently recombined via thiol-Michael addition with different dithiol coupling agents. Compared with the initial ATRP products, the rebuilt polymers have almost the same molecular weight, molecular weight distribution, and chemical composition. Thus, functionality “knock-in” was fulfilled by CM between the model polymer and acrylate followed by recombination using the dithiol compound bearing the desired functionality. Functionality “knock-out” and replacement were accomplished by CM between the model polymer and acrylate followed by recombination using the dithiol compound without and with another functionality, respectively. Owing to the mild conditions and great tolerance of both olefin metathesis and thiol-Michael addition, the present strategy can serve as a useful tool for polymer post-editing through the unique process of chain cleavage (cross metathesis reaction) and recombination (thiol-Michael addition).
C bonds in the polymer chain and acrylates was of high efficiency, resulting in nearly 100% “scissored” chains which were subsequently recombined via thiol-Michael addition with different dithiol coupling agents. Compared with the initial ATRP products, the rebuilt polymers have almost the same molecular weight, molecular weight distribution, and chemical composition. Thus, functionality “knock-in” was fulfilled by CM between the model polymer and acrylate followed by recombination using the dithiol compound bearing the desired functionality. Functionality “knock-out” and replacement were accomplished by CM between the model polymer and acrylate followed by recombination using the dithiol compound without and with another functionality, respectively. Owing to the mild conditions and great tolerance of both olefin metathesis and thiol-Michael addition, the present strategy can serve as a useful tool for polymer post-editing through the unique process of chain cleavage (cross metathesis reaction) and recombination (thiol-Michael addition).
中文翻译:

聚合物链编辑:功能“敲入”,“敲除”以及通过交叉易位反应和硫醇-迈克尔加成的替换
我们在此报告聚合物改性的策略,包括功能性“敲入”,“敲除”和通过烯烃交叉复分解(CM)反应接着硫醇-迈克尔加成反应在聚合物主链上进行取代的操作。使用设计的具有双键和特定官能度的二溴化物引发剂,通过原子转移自由基聚合(ATRP),制备了包含烯烃部分的模型聚合物。
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 聚合物链中的C C键与丙烯酸酯之间的CM反应效率很高,产生了近100%的“剪刀”链,随后这些链通过用不同的二硫醇偶联剂添加硫醇-迈克尔。与最初的ATRP产品相比,重建的聚合物具有几乎相同的分子量,分子量分布和化学组成。因此,通过CM在模型聚合物和丙烯酸酯之间实现官能团“敲入”,然后使用带有所需官能团的二硫醇化合物进行重组。通过模型聚合物和丙烯酸酯之间的CM进行功能“敲除”和替换,然后分别使用不含和具有其他功能的二硫醇化合物进行重组。由于烯烃复分解反应和巯基-迈克尔加成反应的条件温和且耐受性强,
聚合物链中的C C键与丙烯酸酯之间的CM反应效率很高,产生了近100%的“剪刀”链,随后这些链通过用不同的二硫醇偶联剂添加硫醇-迈克尔。与最初的ATRP产品相比,重建的聚合物具有几乎相同的分子量,分子量分布和化学组成。因此,通过CM在模型聚合物和丙烯酸酯之间实现官能团“敲入”,然后使用带有所需官能团的二硫醇化合物进行重组。通过模型聚合物和丙烯酸酯之间的CM进行功能“敲除”和替换,然后分别使用不含和具有其他功能的二硫醇化合物进行重组。由于烯烃复分解反应和巯基-迈克尔加成反应的条件温和且耐受性强,



























 京公网安备 11010802027423号
京公网安备 11010802027423号