当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
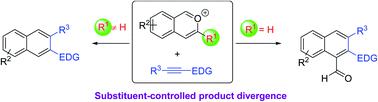
Benzannulation of isobenzopyryliums with electron-rich alkynes: a modular access to β-functionalized naphthalenes
Chemical Science ( IF 8.4 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0sc02502j An Wu 1, 2, 3, 4, 5 , Hui Qian 1, 2, 3, 4, 5 , Wanxiang Zhao 1, 2, 3, 4, 5 , Jianwei Sun 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2020-07-06 , DOI: 10.1039/d0sc02502j An Wu 1, 2, 3, 4, 5 , Hui Qian 1, 2, 3, 4, 5 , Wanxiang Zhao 1, 2, 3, 4, 5 , Jianwei Sun 1, 2, 3, 4, 5
Affiliation
|
Described here is a modular strategy for the rapid synthesis of β-functionalized electron-rich naphthalenes, a family of valuable molecules lacking general access previously. Our approach employs an intermolecular benzannulation of in situ generated isobenzopyrylium ions with various electron-rich alkynes, which were not well utilized for this type of reaction before. These reactions not only feature a broad scope, complete regioselectivity, and mild conditions, but also exhibit unusual product divergence depending on the substrate substitution pattern. This divergence allows further expansion of the product diversity. Control experiments provided preliminary insights into the reaction mechanism.
中文翻译:

异苯并吡啶与富电子炔烃的苯环合反应:模块化获得β-官能化萘
这里描述的是一种模块化策略,用于快速合成β-官能化的富电子萘,这是一类有价值的分子,以前缺乏通用途径。我们的方法采用了原位生成的异苯并吡喃鎓离子与各种富电子炔烃的分子间苯并环化方法,这些方法以前并未很好地用于这种类型的反应。这些反应不仅具有广泛的范围,完全的区域选择性和温和的条件,而且还表现出不同的产物差异,具体取决于底物的取代方式。这种差异允许进一步扩大产品的多样性。对照实验提供了对反应机理的初步见解。
更新日期:2020-08-05
中文翻译:

异苯并吡啶与富电子炔烃的苯环合反应:模块化获得β-官能化萘
这里描述的是一种模块化策略,用于快速合成β-官能化的富电子萘,这是一类有价值的分子,以前缺乏通用途径。我们的方法采用了原位生成的异苯并吡喃鎓离子与各种富电子炔烃的分子间苯并环化方法,这些方法以前并未很好地用于这种类型的反应。这些反应不仅具有广泛的范围,完全的区域选择性和温和的条件,而且还表现出不同的产物差异,具体取决于底物的取代方式。这种差异允许进一步扩大产品的多样性。对照实验提供了对反应机理的初步见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号