当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure of the catalytic subunit of bovine pyruvate dehydrogenase phosphatase.
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-07-06 , DOI: 10.1107/s2053230x20007943 Youzhong Guo 1 , Weihua Qiu 1 , Thomas E Roche 2 , Marvin L Hackert 3
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-07-06 , DOI: 10.1107/s2053230x20007943 Youzhong Guo 1 , Weihua Qiu 1 , Thomas E Roche 2 , Marvin L Hackert 3
Affiliation
|
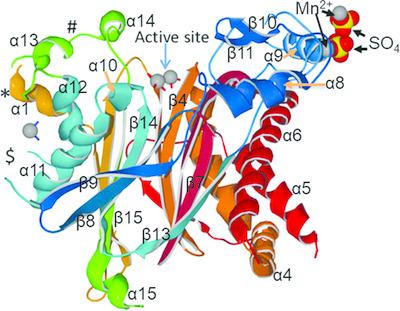
Mammalian pyruvate dehydrogenase (PDH) activity is tightly regulated by phosphorylation and dephosphorylation, which is catalyzed by PDH kinase isomers and PDH phosphatase isomers, respectively. PDH phosphatase isomer 1 (PDP1) is a heterodimer consisting of a catalytic subunit (PDP1c) and a regulatory subunit (PDP1r). Here, the crystal structure of bovine PDP1c determined at 2.1 Å resolution is reported. The crystals belonged to space group P3221, with unit‐cell parameters a = b = 75.3, c = 173.2 Å. The structure was solved by molecular‐replacement methods and refined to a final R factor of 21.9% (Rfree = 24.7%). The final model consists of 402 of a possible 467 amino‐acid residues of the PDP1c monomer, two Mn2+ ions in the active site, an additional Mn2+ ion coordinated by His410 and His414, two MnSO4 ion pairs at special positions near the crystallographic twofold symmetry axis and 226 water molecules. Several new features of the PDP1c structure are revealed. The requirements are described and plausible bases are deduced for the interaction of PDP1c with PDP1r and other components of the pyruvate dehydrogenase complex.
中文翻译:

牛丙酮酸脱氢酶磷酸酶催化亚基的晶体结构。
哺乳动物丙酮酸脱氢酶(PDH)的活性受到磷酸化和去磷酸化的严格调控,磷酸化和去磷酸化分别由PDH激酶异构体和PDH磷酸酶异构体催化。PDH磷酸酶异构体1(PDP1)是由催化亚基(PDP1c)和调节亚基(PDP1r)组成的异二聚体。在此,报告了以2.1分辨率测定的牛PDP1c的晶体结构。晶体属于空间群P 3 2 21,单位晶胞参数a = b = 75.3,c = 173.2Å。通过分子置换方法解决了结构问题,并将其精炼成最终的R因子为21.9%(不含R)= 24.7%)。最终模型由PDP1c单体的467个氨基酸残基中的402个组成,在活性位点中包含两个Mn 2+离子,由His410和His414配位的另一个Mn 2+离子,在靠近特定位置的两个MnSO 4离子对晶体学对称轴和226个水分子。揭示了PDP1c结构的几个新功能。描述了要求,并推论了PDP1c与PDP1r和丙酮酸脱氢酶复合物其他成分相互作用的合理依据。
更新日期:2020-07-06
中文翻译:

牛丙酮酸脱氢酶磷酸酶催化亚基的晶体结构。
哺乳动物丙酮酸脱氢酶(PDH)的活性受到磷酸化和去磷酸化的严格调控,磷酸化和去磷酸化分别由PDH激酶异构体和PDH磷酸酶异构体催化。PDH磷酸酶异构体1(PDP1)是由催化亚基(PDP1c)和调节亚基(PDP1r)组成的异二聚体。在此,报告了以2.1分辨率测定的牛PDP1c的晶体结构。晶体属于空间群P 3 2 21,单位晶胞参数a = b = 75.3,c = 173.2Å。通过分子置换方法解决了结构问题,并将其精炼成最终的R因子为21.9%(不含R)= 24.7%)。最终模型由PDP1c单体的467个氨基酸残基中的402个组成,在活性位点中包含两个Mn 2+离子,由His410和His414配位的另一个Mn 2+离子,在靠近特定位置的两个MnSO 4离子对晶体学对称轴和226个水分子。揭示了PDP1c结构的几个新功能。描述了要求,并推论了PDP1c与PDP1r和丙酮酸脱氢酶复合物其他成分相互作用的合理依据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号