当前位置:
X-MOL 学术
›
Int. J. Energy Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel hexacyanoferrate film coated pencil graphite electrode as sensor and electrode material for environment and energy applications
International Journal of Energy Research ( IF 4.6 ) Pub Date : 2020-07-05 , DOI: 10.1002/er.5640 Sedhu Nagarajan 1 , Vasanthakumar Vasudevan 1 , Theerthagiri Jayaraman 2 , Ramachandran Arumugam 3 , Raj Vairamuthu 1
International Journal of Energy Research ( IF 4.6 ) Pub Date : 2020-07-05 , DOI: 10.1002/er.5640 Sedhu Nagarajan 1 , Vasanthakumar Vasudevan 1 , Theerthagiri Jayaraman 2 , Ramachandran Arumugam 3 , Raj Vairamuthu 1
Affiliation
|
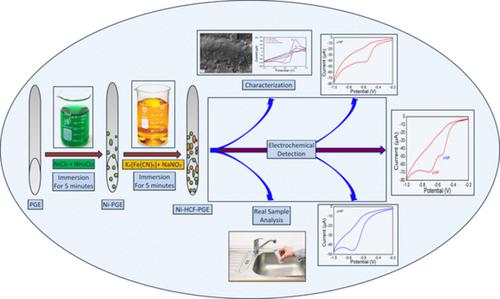
In this present work, a simple electroless deposition method is used for the fabrication of hexacyanoferrate(III)‐nickel deposited pencil graphite (HCF‐Ni‐PGE) electrode and applied as an electrochemical sensor for selective and simultaneous determination of ortho‐ I and para‐ nitrophenol II isomers. The fabricated HCF‐Ni‐PGE was characterized by Fourier transform infrared (FTIR), X‐ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X‐ray (EDX) spectroscopy, and elemental mapping. The electrochemical analysis was carried out using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) techniques in 0.1 M KCl containing K4[Fe(CN)6]. The redox (Fe2+/3+) peak currents were enormously increased due to the successful enhancement in the electroactive surface area of PGE from 0.4 to 1.57 cm2 for HCF‐Ni‐PGE. Further, CV and EIS support the HCF and Ni coating on the PGE surface enhanced the electronic conductivity of HCF‐Ni‐PGE. The electrochemical sensor application of HCF‐Ni‐PGE was analysed using CV and differential pulse voltammetry towards the determination of o‐ and p‐NP in 0.1 M PBS (pH 6.0). The reduction currents of I and II at HCF‐Ni‐PGE was enhanced two times compared with the PGE with better resolution. This, the enhanced activity is due to the positive synergetic effect which increased an electroactive surface area, and electronic conductivity. The limit of detection (LOD) values at HCF‐Ni‐PGE for I and II isomers are 1.98 × 10−7 and 1.43 × 10−7 M with S/N = 3, respectively. The practical applicability of the electrochemical sensor has been investigated in the presence of common interfering agents such as Na+, SO42− and phenol. The simple electroless deposition used for the fabrication of HCF‐Ni‐PGE electrode possessed high surface area and high conductivity, it can also be applicable in the field of energy storage devices of supercapacitor, and battery, fuel cells and solar cells as electrode materials and photocatalysts.
中文翻译:

六氰合铁酸镍薄膜涂层铅笔状石墨电极,作为环境和能源应用的传感器和电极材料
在本工作中,一种简单的化学沉积方法被用于制造六氰基高铁酸盐(III)-镍沉积的铅笔状石墨(HCF-Ni-PGE)电极,并用作电化学传感器,用于选择性和同时测定邻位I和对位-硝基苯酚II异构体。所制造的HCF-Ni-PGE具有傅里叶变换红外(FTIR),X射线衍射(XRD),扫描电子显微镜(SEM),能量色散X射线(EDX)光谱和元素图谱的特征。使用循环伏安法(CV)和电化学阻抗谱(EIS)技术在含K 4 [Fe(CN)6 ]的0.1 M KCl中进行电化学分析。氧化还原(Fe 2 + / 3 +)由于HCF‐Ni‐PGE的PGE电活性表面积成功地从0.4增加到1.57 cm 2,峰值电流大大增加。此外,CV和EIS支持PGE表面上的HCF和Ni涂层,从而增强了HCF-Ni-PGE的电子导电性。使用CV和差分脉冲伏安法分析了HCF-Ni-PGE在电化学传感器中的应用,以确定o-和p‐NP在0.1 M PBS(pH 6.0)中。HCF-Ni-PGE的I和II的还原电流比具有更好分辨率的PGE增强了两倍。这是由于增加了电活性表面积和电子传导性的正协同作用所致。I和II异构体在HCF-Ni-PGE处的检测限(LOD)值分别为S / N = 3时的1.98×10 -7和1.43×10 -7M。已经在常见的干扰剂(例如Na +,SO 4 2-)存在下研究了电化学传感器的实际适用性和苯酚。用于制造HCF-Ni-PGE电极的简单化学沉积具有高的表面积和高的导电性,它还可以应用于超级电容器的储能设备领域,以及作为电极材料的电池,燃料电池和太阳能电池领域。光催化剂。
更新日期:2020-07-05
中文翻译:

六氰合铁酸镍薄膜涂层铅笔状石墨电极,作为环境和能源应用的传感器和电极材料
在本工作中,一种简单的化学沉积方法被用于制造六氰基高铁酸盐(III)-镍沉积的铅笔状石墨(HCF-Ni-PGE)电极,并用作电化学传感器,用于选择性和同时测定邻位I和对位-硝基苯酚II异构体。所制造的HCF-Ni-PGE具有傅里叶变换红外(FTIR),X射线衍射(XRD),扫描电子显微镜(SEM),能量色散X射线(EDX)光谱和元素图谱的特征。使用循环伏安法(CV)和电化学阻抗谱(EIS)技术在含K 4 [Fe(CN)6 ]的0.1 M KCl中进行电化学分析。氧化还原(Fe 2 + / 3 +)由于HCF‐Ni‐PGE的PGE电活性表面积成功地从0.4增加到1.57 cm 2,峰值电流大大增加。此外,CV和EIS支持PGE表面上的HCF和Ni涂层,从而增强了HCF-Ni-PGE的电子导电性。使用CV和差分脉冲伏安法分析了HCF-Ni-PGE在电化学传感器中的应用,以确定o-和p‐NP在0.1 M PBS(pH 6.0)中。HCF-Ni-PGE的I和II的还原电流比具有更好分辨率的PGE增强了两倍。这是由于增加了电活性表面积和电子传导性的正协同作用所致。I和II异构体在HCF-Ni-PGE处的检测限(LOD)值分别为S / N = 3时的1.98×10 -7和1.43×10 -7M。已经在常见的干扰剂(例如Na +,SO 4 2-)存在下研究了电化学传感器的实际适用性和苯酚。用于制造HCF-Ni-PGE电极的简单化学沉积具有高的表面积和高的导电性,它还可以应用于超级电容器的储能设备领域,以及作为电极材料的电池,燃料电池和太阳能电池领域。光催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号