Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.jmgm.2020.107673 Zongsheng Li 1 , Xiulin An 2
|
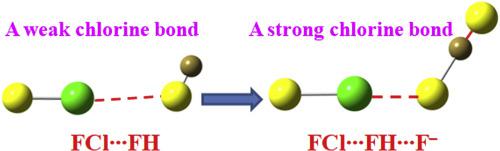
A theoretical calculation has been performed for the ternary complexes XCl∙∙∙FH∙∙∙F− (X = CCH, CN, OH, NC, and F) and the corresponding binary complexes. The halogen bond in the dyad is very weak with the interaction energy less than 2.5 kcal/mol. Interestingly, the halogen bond gets a big enhancement when it combines with a very strong hydrogen bond in FH∙∙∙F−, and the largest interaction energy is up to ∼25.6 kcal/mol in FCl∙∙∙FH∙∙∙F−. The enhancement of halogen bond not only results in a larger elongation of X–Cl bond and a bigger redshift of the bond stretch vibration but also makes the blue-shifting halogen bond in NCCl∙∙∙FH be a red-shifting one in NCCl∙∙∙FH∙∙∙F−. The halogen bond belongs to a purely close-shell interaction in the dyad, while it becomes a partially covalent interaction in XCl∙∙∙FH∙∙∙F− (X = OH, NC, and F) with negative energy density. In FH∙∙∙F−, the proton is shared between the two F atoms, however, this proton transfers towards the F− end in XCl∙∙∙FH∙∙∙F−.
中文翻译:

通过与强氢键和质子转移的协同作用,增强XCl∙∙∙FH∙∙∙F-中的卤素键。
对三元络合物XCl∙∙∙FH∙∙∙F −(X = CCH,CN,OH,NC和F)和相应的二元络合物进行了理论计算。二元体中的卤素键非常弱,相互作用能小于2.5 kcal / mol。有趣的是,卤键得到一个很大的提高,当它与在FH∙∙∙F A非常强的氢键结合- ,和最大的相互作用能达到~25.6千卡/摩尔在FCL∙∙∙FH∙∙∙˚F -。卤素键的增强不仅导致X–Cl键的伸长更大,并且键的拉伸振动更大,而且使NCCl∙∙∙FH中蓝移的卤素键成为NCCl∙中的红移∙∙FH∙∙∙F −。卤素键在二元组中属于纯闭壳相互作用,而在XCl∙∙∙FH∙∙∙F −(X = OH,NC和F)中以负能量密度变为部分共价相互作用。在FH∙∙∙F −中,质子在两个F原子之间共享,但是,该质子在XCl∙∙∙∙FH∙∙∙F −中向F −端转移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号