当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and cytotoxic activity of some new heterocycles incorporating cyclohepta[b]thiophene‐3‐carboxamide derivatives
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-04 , DOI: 10.1002/jhet.4085 Moustafa A. Gouda 1, 2 , Mohammed Al‐Ghorbani 1, 3 , Nabil Al‐Zaqri 4, 5
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-04 , DOI: 10.1002/jhet.4085 Moustafa A. Gouda 1, 2 , Mohammed Al‐Ghorbani 1, 3 , Nabil Al‐Zaqri 4, 5
Affiliation
|
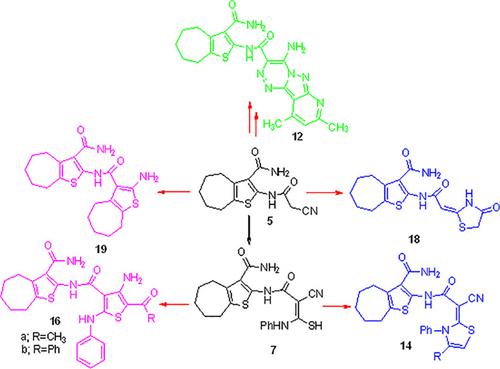
A series of 2‐amino‐5,6,7,8‐tetrahydro‐4H‐cyclohepta[b]thiophene‐3‐carboxamide‐heterocyclic hybrids were synthesized, characterized and their cytotoxic potencies were assessed on four human cell lines. Cyanoacetamide derivative (5) was used as the key synthetic intermediate for the synthesis many derivatives in this study, derivatives 9, 11, 12 were formed by coupled compound 5 with different aryl/heteryl diazonium chlorides, Gewald reaction and Knoevenagel condensation were used for synthesis derivatives 13, 14, 16 by treated cyanoacetamide (5) with different reagents. In another route, compound 5 treated with phenyl isothiocyanate give thiocarbamoyl derivative (7) which used as intermediate underwent oxidative cyclization with different moieties to offer the corresponding thiazoles and thiophene 18, 19, 20, 21, respectively. in vitro cytotoxic activity of prepared compounds were tested against four human tumor cell lines. The result revealed that compound 11a displayed promising cytotoxic activity against HepG2, HCT‐116, MCF‐7, and PC3 cancer cell lines comparing to the positive control (Doxorubicin).
中文翻译:

含有环庚[b]噻吩-3-羧酰胺衍生物的一些新杂环的合成和细胞毒活性
合成了一系列2-氨基-5,6,7,8-四氢-4H-环庚[b]噻吩-3-羧酰胺-杂环杂种,对其特性进行了表征,并在四种人类细胞系中评估了它们的细胞毒性。氰基乙酰胺衍生物(5)被用作用于本研究中的合成许多衍生物中间密钥合成,衍生物9,11,12,通过联接化合物形成5与不同的芳基heteryl重氮氯化物,gewald反应和Knoevenagel缩合中使用/合成衍生物13,14,16通过处理氰基乙酰胺(5)用不同的试剂。在另一条路线中,复合5与处理过的异硫氰酸苯酯给予硫代氨基甲酰基衍生物(7,其用作具有不同的部分中间后行氧化环化,以提供相应的噻唑和噻吩)18,19,20,21,分别。测试了制备的化合物对四种人类肿瘤细胞系的体外细胞毒性活性。结果显示,与阳性对照(阿霉素)相比,化合物11a对HepG2,HCT-116,MCF-7和PC3癌细胞显示出有希望的细胞毒活性。
更新日期:2020-07-04
中文翻译:

含有环庚[b]噻吩-3-羧酰胺衍生物的一些新杂环的合成和细胞毒活性
合成了一系列2-氨基-5,6,7,8-四氢-4H-环庚[b]噻吩-3-羧酰胺-杂环杂种,对其特性进行了表征,并在四种人类细胞系中评估了它们的细胞毒性。氰基乙酰胺衍生物(5)被用作用于本研究中的合成许多衍生物中间密钥合成,衍生物9,11,12,通过联接化合物形成5与不同的芳基heteryl重氮氯化物,gewald反应和Knoevenagel缩合中使用/合成衍生物13,14,16通过处理氰基乙酰胺(5)用不同的试剂。在另一条路线中,复合5与处理过的异硫氰酸苯酯给予硫代氨基甲酰基衍生物(7,其用作具有不同的部分中间后行氧化环化,以提供相应的噻唑和噻吩)18,19,20,21,分别。测试了制备的化合物对四种人类肿瘤细胞系的体外细胞毒性活性。结果显示,与阳性对照(阿霉素)相比,化合物11a对HepG2,HCT-116,MCF-7和PC3癌细胞显示出有希望的细胞毒活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号