当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of stereoisomeric 5-(2-nitro-1-phenylethyl)furan-2(5H)-ones by computation of 1 H and 13 C NMR chemical shifts and electronic circular dichroism spectra
Magnetic Resonance in Chemistry ( IF 2 ) Pub Date : 2020-07-15 , DOI: 10.1002/mrc.5073 Dayane T Lopes 1 , Thomas R Hoye 2 , Elson S Alvarenga 1
Magnetic Resonance in Chemistry ( IF 2 ) Pub Date : 2020-07-15 , DOI: 10.1002/mrc.5073 Dayane T Lopes 1 , Thomas R Hoye 2 , Elson S Alvarenga 1
Affiliation
|
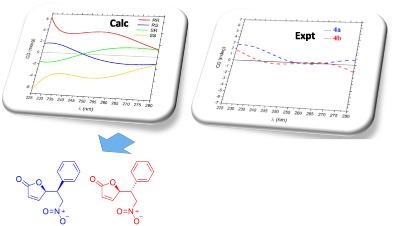
In the present work we describe the preparation of two diastereomers from the enantioselective Michael addition of furan-2(5H)-one to (E)-(2-nitrovinyl)benzene catalyzed by a dinuclear Zn-complex. The relative configurations of the diastereomeric products were assigned by comparing NMR experimental chemical shift data with those computed by DFT methods. Corrected mean absolute error (CMAE) and CP3 analyses were used to compare the data sets. The absolute configuration of each diastereomer was initially assigned by analysis of electronic circular dichroism (ECD) data, which was consistent with that of the known X-ray crystallographic structure of the product of a related reaction, namely (R)-5-((R)-1-(4-chlorophenyl)-2-nitroethyl)furan-2(5H)-one.
中文翻译:

通过计算 1 H 和 13 C NMR 化学位移和电子圆二色光谱表征立体异构 5-(2-硝基-1-苯乙基)呋喃-2(5H)-酮
在目前的工作中,我们描述了在双核锌配合物的催化下,通过呋喃-2(5H)-酮与(E)-(2-硝基乙烯基)苯的对映选择性迈克尔加成来制备两种非对映体。通过将 NMR 实验化学位移数据与 DFT 方法计算的数据进行比较,确定非对映体产物的相对构型。使用校正平均绝对误差 (CMAE) 和 CP3 分析来比较数据集。每个非对映异构体的绝对构型最初是通过电子圆二色性(ECD)数据分析确定的,这与相关反应产物的已知X射线晶体结构一致,即(R)-5-(( R)-1-(4-氯苯基)-2-硝基乙基)呋喃-2(5H)-一。
更新日期:2020-07-15
中文翻译:

通过计算 1 H 和 13 C NMR 化学位移和电子圆二色光谱表征立体异构 5-(2-硝基-1-苯乙基)呋喃-2(5H)-酮
在目前的工作中,我们描述了在双核锌配合物的催化下,通过呋喃-2(5H)-酮与(E)-(2-硝基乙烯基)苯的对映选择性迈克尔加成来制备两种非对映体。通过将 NMR 实验化学位移数据与 DFT 方法计算的数据进行比较,确定非对映体产物的相对构型。使用校正平均绝对误差 (CMAE) 和 CP3 分析来比较数据集。每个非对映异构体的绝对构型最初是通过电子圆二色性(ECD)数据分析确定的,这与相关反应产物的已知X射线晶体结构一致,即(R)-5-(( R)-1-(4-氯苯基)-2-硝基乙基)呋喃-2(5H)-一。


























 京公网安备 11010802027423号
京公网安备 11010802027423号