Journal of Proteomics ( IF 3.3 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.jprot.2020.103893 Amutha Periyasamy 1 , Gopal Gopisetty 1 , Malliga Joyimallaya Subramanium 2 , Sridevi Velusamy 3 , Thangarajan Rajkumar 1
|
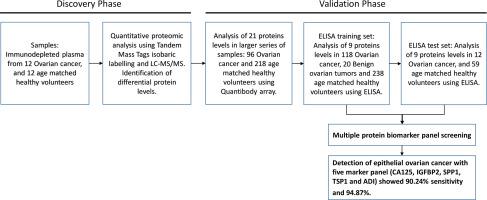
Diagnosis of Ovarian cancer (OC) has been a challenge, the purpose, therefore is to identify plasma proteins differentially expressed in epithelial ovarian cancer patients. Human plasma samples from patients with OC (n = 138), benign tumors (n = 20) and controls (n = 238) were used. Tandem Mass Tag (TMT) based quantitative analysis by high resolution mass spectrometry, was followed by validation using Quantibody array and ELISA techniques. 507 plasma proteins showed differential protein levels in OC plasma samples. 21 proteins were validated using Quantibody array. Further, nine proteins (CA125, CFD, CST3, ICAM1, IGFBP2, IGFBP3, SPP1, TSP1 and VEGFA) which showed significant differences in protein levels in Quantibody array analysis were validated using ELISA. In ELISA, the levels of CA125, IGFBP2, ICAM1 and SPP1 were significantly increased and levels of Adipsin and TSP1 were decreased in tumors compared to controls and benign group. Epithelial ovarian cancer diagnosis model combining five markers (CA125, IGFBP2, SPP1, TSP1 and ADI) showed 90.24% sensitivity and 94.87% specificity. In conclusion a panel of 5 plasma proteins has been found to be useful in distinguishing plasma samples from epithelial ovarian cancers from patients with benign tumors and healthy normal subjects. This has the potential as a diagnostic assay for epithelial ovarian cancer.
Significance
The significance of this case-control study is based on the large and well defined ovarian cancer patient population (epithelial ovarian cancers including serous and mucinous subtypes), age matched controls and benign ovarian tumors. This study incorporates a discovery phase involving quantitative proteomic analysis of immune-depleted plasma followed by two levels of validation studies involving a selected list of proteins using antibody arrays and ELISA. The validations were performed on an independent set of samples comprising of epithelial ovarian cancer subtypes, controls and benign tumors. The multiple marker combination comprising of Adipsin, CA125, IGFBP2, SPP1 and TSP1 identified in the study by ELISA could enable rapid translation to a larger screening study.
中文翻译:

上皮性卵巢癌中差异血浆蛋白水平的鉴定和验证。
卵巢癌(OC)的诊断一直是一个挑战,其目的是鉴定在上皮性卵巢癌患者中差异表达的血浆蛋白。患有OC(n = 138),良性肿瘤(n = 20)和对照(n = 238)。通过高分辨率质谱法进行基于串联质谱标签(TMT)的定量分析,然后使用Quantibody阵列和ELISA技术进行验证。507种血浆蛋白在OC血浆样品中显示出不同的蛋白水平。使用Quantibody阵列验证了21种蛋白质。此外,使用ELISA验证了在Quantibody阵列分析中显示出显着差异的九种蛋白质(CA125,CFD,CST3,ICAM1,IGFBP2,IGFBP3,SPP1,TSP1和VEGFA)。在ELISA中,与对照组和良性组相比,肿瘤中CA125,IGFBP2,ICAM1和SPP1的水平显着增加,而脂肪酶和TSP1的水平则下降。结合五个标志物(CA125,IGFBP2,SPP1,TSP1和ADI)的上皮性卵巢癌诊断模型显示出90.24%的敏感性和94.87%的特异性。总之,已发现一组5种血浆蛋白可用于区分血浆样品与上皮性卵巢癌与良性肿瘤患者和健康正常人。这有可能作为上皮性卵巢癌的诊断方法。
意义
这项病例对照研究的意义是基于大量且定义明确的卵巢癌患者人群(包括浆液和粘液亚型的上皮性卵巢癌),年龄匹配的对照和良性卵巢肿瘤。这项研究包括一个发现阶段,涉及对免疫耗竭血浆进行定量蛋白质组学分析,然后进行两个级别的验证研究,其中涉及使用抗体阵列和ELISA筛选蛋白质的选定列表。验证是在一组独立的样品上进行的,这些样品包括上皮性卵巢癌亚型,对照和良性肿瘤。通过ELISA在研究中鉴定的包含Adipsin,CA125,IGFBP2,SPP1和TSP1的多标记组合可以使快速翻译成更大的筛选研究。

























 京公网安备 11010802027423号
京公网安备 11010802027423号