当前位置:
X-MOL 学术
›
J. Pharm. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pharmacokinetic comparison with different assays for simultaneous determination of cis-, trans-cefprozil diastereomers in human plasma
Journal of Pharmaceutical Analysis ( IF 8.8 ) Pub Date : 2020-07-05 , DOI: 10.1016/j.jpha.2020.07.001 Seung-Hyun Jeong 1 , Ji-Hun Jang 1 , Hea-Young Cho 2 , Yong-Bok Lee 1
Journal of Pharmaceutical Analysis ( IF 8.8 ) Pub Date : 2020-07-05 , DOI: 10.1016/j.jpha.2020.07.001 Seung-Hyun Jeong 1 , Ji-Hun Jang 1 , Hea-Young Cho 2 , Yong-Bok Lee 1
Affiliation
|
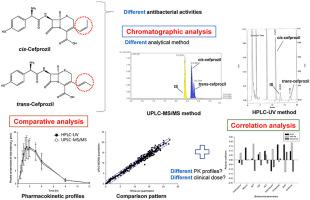
The purpose of this study was to compare pharmacokinetic (PK) parameters obtained using two newly developed assays, HPLC-UV and UPLC-ESI-MS/MS. Selection of assay and results obtained therefrom are very important in PK studies and can have a major impact on the PK-based clinical dose and usage settings. For this study, we developed two new methods that are most commonly used in biosample analysis and focused on PK parameters obtained from them. By HPLC-UV equipped with a Luna-C column using UV detector, cefprozil diastereomers were separated using water containing 2% () acetic acid and acetonitrile as a mobile phase. By UPLC-ESI-MS/MS equipped with a HALO-Ccolumn, cefprozil diastereomers were separated using 0.5% ( aqueous formic acid containing 5 mM ammonium-formate buffer and methanol as a mobile phase. Chromatograms showed high resolution, sensitivity, and selectivity without interference by plasma constituents. Both intra- and inter-day precisions (CV, %) were within 8.88% for HPLC-UV and UPLC-ESI-MS/MS. Accuracy of both methods was 95.67%–107.50%. These two analytical methods satisfied the criteria of international guidance and could be successfully applied to PK study. Comparison of PK parameters between two assays confirmed that there is a difference in the predicted minimum plasma concentrations at steady state, which may affect clinical dose and usage settings. Furthermore, we confirmed possible correlation between PK parameters and various biochemical parameters after oral administration of 1000 mg cefprozil to humans.
中文翻译:

同时测定人血浆中顺式、反式头孢丙烯非对映体的不同测定法的药代动力学比较
本研究的目的是比较使用两种新开发的检测方法(HPLC-UV 和 UPLC-ESI-MS/MS)获得的药代动力学 (PK) 参数。测定方法的选择以及从中获得的结果在 PK 研究中非常重要,并且可能对基于 PK 的临床剂量和使用设置产生重大影响。在本研究中,我们开发了两种生物样本分析中最常用的新方法,并重点关注从中获得的 PK 参数。通过配备Luna-C柱并使用UV检测器的HPLC-UV,使用含有2%()乙酸的水和乙腈作为流动相分离头孢丙烯非对映体。通过配备 HALO-C 柱的 UPLC-ESI-MS/MS,使用含有 5 mM 甲酸铵缓冲液的 0.5% ( 甲酸水溶液和甲醇作为流动相) 分离头孢丙烯非对映异构体。色谱图显示出高分辨率、灵敏度和选择性,无需HPLC-UV 和 UPLC-ESI-MS/MS 的日内和日间精密度 (CV, %) 均在 8.88% 以内,这两种分析方法的准确度均为 95.67%–107.50%。满足国际指导标准,可以成功应用于 PK 研究 两种测定之间的 PK 参数比较证实,稳态时的预测最低血浆浓度存在差异,这可能会影响临床剂量和使用设置。证实人体口服 1000 mg 头孢丙烯后 PK 参数与各种生化参数之间可能存在相关性。
更新日期:2020-07-05
中文翻译:

同时测定人血浆中顺式、反式头孢丙烯非对映体的不同测定法的药代动力学比较
本研究的目的是比较使用两种新开发的检测方法(HPLC-UV 和 UPLC-ESI-MS/MS)获得的药代动力学 (PK) 参数。测定方法的选择以及从中获得的结果在 PK 研究中非常重要,并且可能对基于 PK 的临床剂量和使用设置产生重大影响。在本研究中,我们开发了两种生物样本分析中最常用的新方法,并重点关注从中获得的 PK 参数。通过配备Luna-C柱并使用UV检测器的HPLC-UV,使用含有2%()乙酸的水和乙腈作为流动相分离头孢丙烯非对映体。通过配备 HALO-C 柱的 UPLC-ESI-MS/MS,使用含有 5 mM 甲酸铵缓冲液的 0.5% ( 甲酸水溶液和甲醇作为流动相) 分离头孢丙烯非对映异构体。色谱图显示出高分辨率、灵敏度和选择性,无需HPLC-UV 和 UPLC-ESI-MS/MS 的日内和日间精密度 (CV, %) 均在 8.88% 以内,这两种分析方法的准确度均为 95.67%–107.50%。满足国际指导标准,可以成功应用于 PK 研究 两种测定之间的 PK 参数比较证实,稳态时的预测最低血浆浓度存在差异,这可能会影响临床剂量和使用设置。证实人体口服 1000 mg 头孢丙烯后 PK 参数与各种生化参数之间可能存在相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号