Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Autoignition of diethyl ether and a diethyl ether/ethanol blend
Fuel ( IF 7.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fuel.2020.118553 Gani Issayev , S. Mani Sarathy , Aamir Farooq
Fuel ( IF 7.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fuel.2020.118553 Gani Issayev , S. Mani Sarathy , Aamir Farooq
|
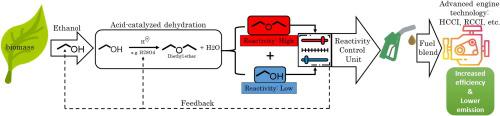
Abstract Binary blends of fast-reacting diethyl ether (DEE) and slow-reacting ethanol (EtOH) are quite promising as renewable replacements for conventional fuels in modern compression ignition engines. In this work, pure diethyl ether and a 50/50 M binary blend of diethyl ether and ethanol (DEE/EtOH) were investigated in a shock tube and a rapid compression machine. Ignition delay times were measured over the temperature range of 550–1000 K, pressures of 20–40 bar, and equivalence ratios of 0.5–1. Literature reaction mechanisms of diethyl ether and ethanol were combined to simulate the reactivity trends of the blends. Species rate-of-production and sensitivity analyses were performed to analyze the interplay between radicals originating from the two fuels. Multistage ignition behavior was observed in both experiments and simulations, with peculiar 3-stage ignition visible at fuel-lean conditions. Kinetic analyses were used to identify the reactions controlling various stages of ignition. Reactivity comparison of DEE/EtOH and dimethyl ether/ethanol (DME/EtOH) blends showed that the oxidation of DEE blends is controlled by acetaldehyde whereas formaldehyde controls the oxidation of DME blends.
中文翻译:

乙醚和乙醚/乙醇混合物的自燃
摘要 快反应乙醚 (DEE) 和慢反应乙醇 (EtOH) 的二元混合物作为现代压燃发动机中传统燃料的可再生替代品非常有前途。在这项工作中,在冲击管和快速压缩机中研究了纯乙醚和 50/50 M 乙醚和乙醇的二元混合物 (DEE/EtOH)。点火延迟时间是在 550-1000 K 的温度范围、20-40 bar 的压力和 0.5-1 的当量比下测量的。结合二乙醚和乙醇的文献反应机理来模拟共混物的反应趋势。进行了物种产生率和敏感性分析,以分析源自两种燃料的自由基之间的相互作用。在实验和模拟中都观察到了多级点火行为,在贫油条件下可见特殊的 3 级点火。动力学分析用于确定控制点火各个阶段的反应。DEE/EtOH 和二甲醚/乙醇 (DME/EtOH) 混合物的反应性比较表明,DEE 混合物的氧化受乙醛控制,而甲醛控制 DME 混合物的氧化。
更新日期:2020-11-01
中文翻译:

乙醚和乙醚/乙醇混合物的自燃
摘要 快反应乙醚 (DEE) 和慢反应乙醇 (EtOH) 的二元混合物作为现代压燃发动机中传统燃料的可再生替代品非常有前途。在这项工作中,在冲击管和快速压缩机中研究了纯乙醚和 50/50 M 乙醚和乙醇的二元混合物 (DEE/EtOH)。点火延迟时间是在 550-1000 K 的温度范围、20-40 bar 的压力和 0.5-1 的当量比下测量的。结合二乙醚和乙醇的文献反应机理来模拟共混物的反应趋势。进行了物种产生率和敏感性分析,以分析源自两种燃料的自由基之间的相互作用。在实验和模拟中都观察到了多级点火行为,在贫油条件下可见特殊的 3 级点火。动力学分析用于确定控制点火各个阶段的反应。DEE/EtOH 和二甲醚/乙醇 (DME/EtOH) 混合物的反应性比较表明,DEE 混合物的氧化受乙醛控制,而甲醛控制 DME 混合物的氧化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号