当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Depression effect of Al(Ⅲ) and Fe(Ⅲ) on rutile flotation using dodecylamine polyxyethylene ether as collector
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.colsurfa.2020.125269 Qian Chen , Maomao Tian , Richard M. Kasomo , Hongqiang Li , Huifang Zheng , Shaoxian Song , Huihua Luo , Dongsheng He
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.colsurfa.2020.125269 Qian Chen , Maomao Tian , Richard M. Kasomo , Hongqiang Li , Huifang Zheng , Shaoxian Song , Huihua Luo , Dongsheng He
|
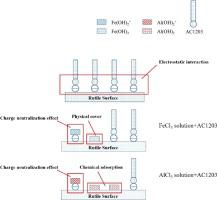
Abstract Few literatures pay emphasis on investigating the effect brought out by metal ions on flotation in the case of cationic collector. In this work, the effect of Al(Ⅲ) and Fe(Ⅲ) on rutile floatability using dodecylamine polyxyethylene ether (AC1203) as collector was evaluated. In addition to the flotation tests, zeta potential measurements, solution chemistry calculation, surface tension measurement and XPS analysis were conducted to elucidate the detailed depression mechanism. The results indicate that the floatability of rutile seriously inhibited by Al(Ⅲ) and Fe(Ⅲ), and the depression effect of Al(Ⅲ) is much stronger than that of Fe(Ⅲ). Moreover, on the basis of zeta potential and solution chemistry calculation tests, Al(OH)3 and Fe(OH)3 precipitates are believed to be the principal components that act with rutile surface, and the preferential adsorption of Al(OH)3 precipitate could further deteriorate the interaction of collector with rutile. It is worth mentioning that Al(Ⅲ) and Fe(Ⅲ) are capable of acting with AC1203 to form complexes, consequently affecting the flotation environment. Furthermore, XPS analysis reveals that Fe(OH)3 precipitate tend to adsorb on rutile surface through physical interaction, while Al(OH)3 precipitate could react with Os and bridging O on rutile surface to form Ti-O-Al bonds, resulting in less active Ti sites for collector adsorption.
中文翻译:

Al(Ⅲ)和Fe(Ⅲ)对十二胺聚氧乙烯醚作捕收剂浮选金红石的抑制作用
摘要 在阳离子捕收剂的情况下,很少有文献着重研究金属离子对浮选的影响。本工作以十二胺聚氧乙烯醚(AC1203)为捕收剂,评价了Al(Ⅲ)和Fe(Ⅲ)对金红石可浮性的影响。除了浮选试验外,还进行了 zeta 电位测量、溶液化学计算、表面张力测量和 XPS 分析,以阐明详细的抑制机制。结果表明,Al(Ⅲ)和Fe(Ⅲ)严重抑制了金红石的可浮性,且Al(Ⅲ)的抑制作用远强于Fe(Ⅲ)。此外,根据zeta电位和溶液化学计算测试,Al(OH)3和Fe(OH)3沉淀被认为是与金红石表面作用的主要成分,Al(OH)3 沉淀的优先吸附会进一步恶化集电体与金红石的相互作用。值得一提的是,Al(Ⅲ)和Fe(Ⅲ)能够与AC12O3作用形成配合物,从而影响浮选环境。此外,XPS 分析表明 Fe(OH)3 沉淀倾向于通过物理相互作用吸附在金红石表面,而 Al(OH)3 沉淀可以与 Os 反应并在金红石表面架桥 O 形成 Ti-O-Al 键,导致用于收集器吸附的活性钛位点较少。
更新日期:2020-10-01
中文翻译:

Al(Ⅲ)和Fe(Ⅲ)对十二胺聚氧乙烯醚作捕收剂浮选金红石的抑制作用
摘要 在阳离子捕收剂的情况下,很少有文献着重研究金属离子对浮选的影响。本工作以十二胺聚氧乙烯醚(AC1203)为捕收剂,评价了Al(Ⅲ)和Fe(Ⅲ)对金红石可浮性的影响。除了浮选试验外,还进行了 zeta 电位测量、溶液化学计算、表面张力测量和 XPS 分析,以阐明详细的抑制机制。结果表明,Al(Ⅲ)和Fe(Ⅲ)严重抑制了金红石的可浮性,且Al(Ⅲ)的抑制作用远强于Fe(Ⅲ)。此外,根据zeta电位和溶液化学计算测试,Al(OH)3和Fe(OH)3沉淀被认为是与金红石表面作用的主要成分,Al(OH)3 沉淀的优先吸附会进一步恶化集电体与金红石的相互作用。值得一提的是,Al(Ⅲ)和Fe(Ⅲ)能够与AC12O3作用形成配合物,从而影响浮选环境。此外,XPS 分析表明 Fe(OH)3 沉淀倾向于通过物理相互作用吸附在金红石表面,而 Al(OH)3 沉淀可以与 Os 反应并在金红石表面架桥 O 形成 Ti-O-Al 键,导致用于收集器吸附的活性钛位点较少。



























 京公网安备 11010802027423号
京公网安备 11010802027423号