当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Resolving the mystery of the second charge reversal on solid surfaces in the presence of divalent heavy metal ions
Applied Surface Science ( IF 6.7 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apsusc.2020.147128 Foad Raji , Majid Ejtemaei , Anh.V. Nguyen
Applied Surface Science ( IF 6.7 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apsusc.2020.147128 Foad Raji , Majid Ejtemaei , Anh.V. Nguyen
|
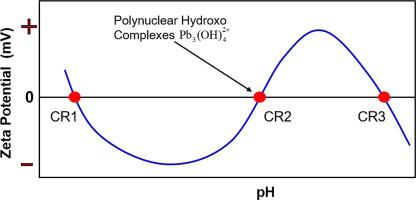
Abstract Understanding adsorption of heavy metal ions onto solid surfaces is critical to many areas but remains limited due to complicated surface charge reversals (CRs). Experiments show three distinctive pHs values of CRs occurring during heavy metal ion interactions with oxide minerals like silica. CR1 and CR3 are well understood. Mechanisms for CR2 are mysteriously controversial. Here we have resolved the mystery by investigating specific adsorption and surface precipitation on the CR2 formation. We determined electrokinetics of silica surfaces versus pH and surface coverage of adsorbed species in the presence of Pb(II), Cu(II), Zn(II), Mg(II), and Ca(II) and quantified the adsorbed chemical species by X-ray photoelectron spectroscopy. For Pb(II), polynuclear complexes like P b 3 ( O H ) 4 2 + are critical to the CR2 formation at full surface coverage. The polynuclear hydroxo complexes (large and less hydrated) with strong affinity to the silica surface penetrate the Stern layer to form the inner-sphere complexation with the surface and reverse its charge. The adsorption of unhydrolyzed cations and their first hydrolysis product compress electrical double layer and neutralize the charge but cannot reverse its sign. Our results shed light on the critical role of polynuclear complexes of heavy metal ions for the CR2 formation in many practical applications.
中文翻译:

解开二价重金属离子存在下固体表面二次电荷反转之谜
摘要 了解重金属离子在固体表面上的吸附对许多领域至关重要,但由于复杂的表面电荷反转 (CRs) 而仍然受到限制。实验表明,在重金属离子与氧化矿物(如二氧化硅)相互作用期间,CRs 的三个不同 pH 值。CR1 和 CR3 很好理解。CR2 的机制具有神秘的争议性。在这里,我们通过研究 CR2 形成的特定吸附和表面沉淀来解决这个谜团。我们确定了在 Pb(II)、Cu(II)、Zn(II)、Mg(II) 和 Ca(II) 存在下,二氧化硅表面的电动力学与 pH 值和吸附物质的表面覆盖率的关系,并通过以下方式量化了吸附的化学物质X 射线光电子能谱。对于 Pb(II),P b 3 (OH) 4 2 + 等多核配合物对于全表面覆盖的 CR2 形成至关重要。对二氧化硅表面具有强亲和力的多核羟基络合物(大且水合较少)穿透 Stern 层与表面形成内球络合物并反转其电荷。未水解的阳离子及其第一个水解产物的吸附会压缩双电层并中和电荷,但不能反转其符号。我们的结果阐明了重金属离子的多核配合物在许多实际应用中对 CR2 形成的关键作用。未水解的阳离子及其第一个水解产物的吸附会压缩双电层并中和电荷,但不能反转其符号。我们的结果阐明了重金属离子的多核配合物在许多实际应用中对 CR2 形成的关键作用。未水解的阳离子及其第一个水解产物的吸附会压缩双电层并中和电荷,但不能反转其符号。我们的结果阐明了重金属离子的多核配合物在许多实际应用中对 CR2 形成的关键作用。
更新日期:2020-11-01
中文翻译:

解开二价重金属离子存在下固体表面二次电荷反转之谜
摘要 了解重金属离子在固体表面上的吸附对许多领域至关重要,但由于复杂的表面电荷反转 (CRs) 而仍然受到限制。实验表明,在重金属离子与氧化矿物(如二氧化硅)相互作用期间,CRs 的三个不同 pH 值。CR1 和 CR3 很好理解。CR2 的机制具有神秘的争议性。在这里,我们通过研究 CR2 形成的特定吸附和表面沉淀来解决这个谜团。我们确定了在 Pb(II)、Cu(II)、Zn(II)、Mg(II) 和 Ca(II) 存在下,二氧化硅表面的电动力学与 pH 值和吸附物质的表面覆盖率的关系,并通过以下方式量化了吸附的化学物质X 射线光电子能谱。对于 Pb(II),P b 3 (OH) 4 2 + 等多核配合物对于全表面覆盖的 CR2 形成至关重要。对二氧化硅表面具有强亲和力的多核羟基络合物(大且水合较少)穿透 Stern 层与表面形成内球络合物并反转其电荷。未水解的阳离子及其第一个水解产物的吸附会压缩双电层并中和电荷,但不能反转其符号。我们的结果阐明了重金属离子的多核配合物在许多实际应用中对 CR2 形成的关键作用。未水解的阳离子及其第一个水解产物的吸附会压缩双电层并中和电荷,但不能反转其符号。我们的结果阐明了重金属离子的多核配合物在许多实际应用中对 CR2 形成的关键作用。未水解的阳离子及其第一个水解产物的吸附会压缩双电层并中和电荷,但不能反转其符号。我们的结果阐明了重金属离子的多核配合物在许多实际应用中对 CR2 形成的关键作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号