Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.apcatb.2020.119305 Yu Kang , Yujia Han , Ming Tian , Chuande Huang , Chaojie Wang , Jian Lin , Baolin Hou , Yang Su , Lin Li , Junhu Wang , Xiaodong Wang
|
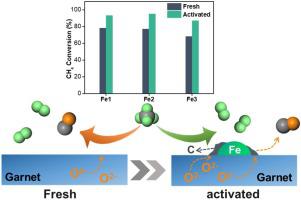
Fe-based oxygen carries (OCs) have attracted wide attention due to the low cost and environmental compatibility for chemical looping methane partial oxidation. However, they usually suffer from low CH4 reactivity mainly due to insufficient synergy between catalytic CH4 activation and lattice oxygen mobility. In this work, Fe ions with different amount were incorporated into a novel garnet structure (Y3FexAl5-xO12), which exhibited drastically increased CH4 conversion with reduction time. This resulted from in-situ formed Fe0 sites at the early stage of reduction, which led to a more efficient reaction route involving methane catalytic decomposition on Fe0 and the resulted carbon oxidation easier by lattice oxygen, compared with methane directly oxidized by OCs. Moreover, the amount of Fe ions in garnet influenced surface exposed Fe0 sites and oxygen mobility, inducing Y3Fe2Al3O12 performed the best with almost 94 % methane conversion thanks to the combined advantages of larger surface exposed Fe0 and higher lattice oxygen mobility.
中文翻译:

通过化学循环,将铁基石榴石上的甲烷转化为合成气
铁基载氧体(OCs)由于其低成本和对化学环甲烷部分氧化的环境兼容性而受到广泛关注。然而,它们通常遭受低CH 4反应性的主要原因是催化CH 4活化和晶格氧迁移率之间的协同作用不足。在这项工作中,不同数量的Fe离子被掺入一种新型的石榴石结构(Y 3 Fe x Al 5-x O 12)中,随着还原时间的增加,其CH 4转化率急剧增加。这是由于原位形成的Fe 0在还原的早期阶段,这导致了更有效的反应路线,其中包括甲烷催化的Fe 0催化分解,以及与OCs直接氧化的甲烷相比,晶格氧更容易导致碳氧化。此外,石榴石中的Fe离子数量影响了表面暴露的Fe 0位和氧迁移率,诱导Y 3 Fe 2 Al 3 O 12表现最佳,甲烷转化率几乎达到94%,这归因于更大的表面暴露Fe 0和更高的综合优势。晶格氧迁移率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号