当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A cationic Zr-based metal organic framework with enhanced acidic resistance for selective and efficient removal of CrO42−
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-07-03 , DOI: 10.1039/d0nj02279a Chao Jiang 1, 2, 3, 4, 5 , Ruopei Sun 1, 2, 3, 4, 5 , Ziyao Du 1, 2, 3, 4, 5 , Vikramjeet Singh 6, 7, 8, 9, 10 , Suwen Chen 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-07-03 , DOI: 10.1039/d0nj02279a Chao Jiang 1, 2, 3, 4, 5 , Ruopei Sun 1, 2, 3, 4, 5 , Ziyao Du 1, 2, 3, 4, 5 , Vikramjeet Singh 6, 7, 8, 9, 10 , Suwen Chen 1, 2, 3, 4, 5
Affiliation
|
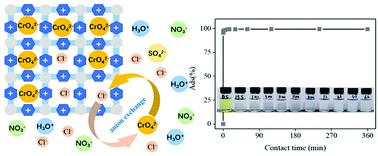
Water pollution caused by CrO42− has become a global concern due to its high aqueous solubility and good mobility in the underground environment. Recently, water stable zirconium based metal–organic frameworks (Zr-MOFs) have been tested as adsorbents to remove pollutants from contaminated water. However, the low adsorption capacity of Zr-MOFs towards ionic pollutants is unsatisfactory due to their neutral nature. Herein, a cationic Zr-MOF (Zr-C-MOF) was obtained by directly introducing a pyridinium salt as a ligand with an impressive ion exchange capability. Zr-C-MOF demonstrated enhanced acidic resistance and selectively high adsorption of CrO42−. The characteristics of the obtained Zr-C-MOF were confirmed via various techniques including PXRD, FT-IR spectroscopy, 1H-NMR spectroscopy, TGA, SEM, EDX and XPS. Batch adsorption studies have been conducted to gain a deep understanding of the kinetics mechanism, pH effects, adsorption isotherm and the effects of other competing ions. The kinetics and adsorption isotherm of CrO42− adsorption onto Zr-C-MOF were found to well fit the pseudo-second-order rate equation and the Langmuir model, respectively. The comparison of FT-IR, PXRD, SEM, EDX and XPS results of the samples before and after CrO42− adsorption revealed the adsorption mechanism of the anion exchange process.
中文翻译:

阳离子Zr基金属有机骨架,具有增强的耐酸性,可选择性有效地去除CrO42-
由于CrO 4 2-的高水溶性和在地下环境中的良好迁移性,已引起全球关注。最近,已经测试了水稳定性锆基金属-有机骨架(Zr-MOF)作为吸附剂,以去除污染水中的污染物。但是,Zr-MOF对离子污染物的低吸附能力由于其中性而不能令人满意。在此,通过直接引入吡啶鎓盐作为具有令人印象深刻的离子交换能力的配体而获得阳离子Zr-MOF(Zr-C-MOF)。Zr-C-MOF表现出增强的耐酸性和对CrO 4 2-的选择性高吸附。所得的Zr-C-MOF的特征通过各种技术,包括PXRD,FT-IR光谱,1 H-NMR光谱,TGA,SEM,EDX和XPS。进行了批量吸附研究,以深入了解动力学机理,pH值影响,吸附等温线以及其他竞争离子的影响。的CrO的动力学和吸附等温线4 2-吸附到的Zr-C-MOF被发现很好适应伪二阶速率方程和Langmuir模型,分别。CrO 4 2-吸附前后样品的FT-IR,PXRD,SEM,EDX和XPS结果的比较揭示了阴离子交换过程的吸附机理。
更新日期:2020-07-27
中文翻译:

阳离子Zr基金属有机骨架,具有增强的耐酸性,可选择性有效地去除CrO42-
由于CrO 4 2-的高水溶性和在地下环境中的良好迁移性,已引起全球关注。最近,已经测试了水稳定性锆基金属-有机骨架(Zr-MOF)作为吸附剂,以去除污染水中的污染物。但是,Zr-MOF对离子污染物的低吸附能力由于其中性而不能令人满意。在此,通过直接引入吡啶鎓盐作为具有令人印象深刻的离子交换能力的配体而获得阳离子Zr-MOF(Zr-C-MOF)。Zr-C-MOF表现出增强的耐酸性和对CrO 4 2-的选择性高吸附。所得的Zr-C-MOF的特征通过各种技术,包括PXRD,FT-IR光谱,1 H-NMR光谱,TGA,SEM,EDX和XPS。进行了批量吸附研究,以深入了解动力学机理,pH值影响,吸附等温线以及其他竞争离子的影响。的CrO的动力学和吸附等温线4 2-吸附到的Zr-C-MOF被发现很好适应伪二阶速率方程和Langmuir模型,分别。CrO 4 2-吸附前后样品的FT-IR,PXRD,SEM,EDX和XPS结果的比较揭示了阴离子交换过程的吸附机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号