当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isobaric Binary Vapor–Liquid Equilibrium of Ethanol + Glycerol and 1-Propanol + Glycerol Systems at 16.0 and 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-07-03 , DOI: 10.1021/acs.jced.0c00089 Moh Arif Batutah 1 , Kuswandi Kuswandi 1 , Gede Wibawa 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-07-03 , DOI: 10.1021/acs.jced.0c00089 Moh Arif Batutah 1 , Kuswandi Kuswandi 1 , Gede Wibawa 1
Affiliation
|
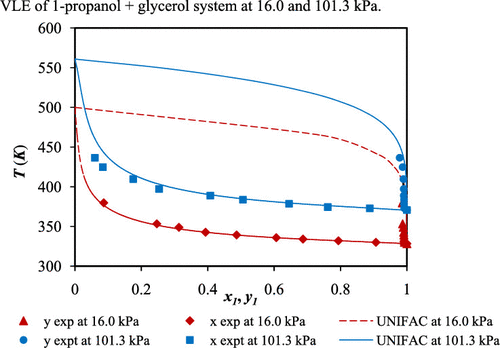
The isobaric binary vapor–liquid equilibrium data of ethanol + glycerol and 1-propanol + glycerol systems were collected experimentally at two pressure levels of 16.0 and 101.3 kPa using modified Glass Othmer-Still. Refractometer index Bausch and Lomb ABBE-type was used to analyze the equilibrium samples of liquid and vapor phase compositions. The reliability of the experimental data determined in this study was conformed to the L–W thermodynamic consistency test method proposed by Wisniak ( Ind. Eng. Chem. Res. 1993, 32(7), 1531-1533). The data were regressed with the Wilson, NRTL, and UNIQUAC equations. The deviations of temperatures and vapor phase compositions were given, and the results indicated that the average absolute deviations in temperatures and in vapor phase compositions were less than 0.5 and 1.1%, respectively, for both systems studied. Furthermore, the performance of predictive UNIFAC equation was found to be better at a pressure of 16 kPa than that of 101.3 kPa.
中文翻译:

乙醇+甘油和1-丙醇+甘油系统在16.0和101.3 kPa时的等压二元汽-液平衡
使用改良的Glass Othmer-Still,在16.0 kPa和101.3 kPa两种压力水平下,通过实验收集了乙醇+甘油和1-丙醇+甘油系统的等压二元气液平衡数据。折光率指数Bausch和Lomb ABBE型用于分析液相和气相组成的平衡样品。在这项研究中所确定的实验数据的可靠性被适形于大号- w ^由Wisniak(提议的热力学一致性测试方法工业工程化学水库。 1993年,32(7),1531-1533)。用Wilson,NRTL和UNIQUAC方程对数据进行回归。给出了温度和气相组成的偏差,结果表明,对于所研究的两个系统,温度和气相组成的平均绝对偏差分别小于0.5%和1.1%。此外,发现在压力为16 kPa时,预测UNIFAC方程的性能要好于101.3 kPa。
更新日期:2020-08-14
中文翻译:

乙醇+甘油和1-丙醇+甘油系统在16.0和101.3 kPa时的等压二元汽-液平衡
使用改良的Glass Othmer-Still,在16.0 kPa和101.3 kPa两种压力水平下,通过实验收集了乙醇+甘油和1-丙醇+甘油系统的等压二元气液平衡数据。折光率指数Bausch和Lomb ABBE型用于分析液相和气相组成的平衡样品。在这项研究中所确定的实验数据的可靠性被适形于大号- w ^由Wisniak(提议的热力学一致性测试方法工业工程化学水库。 1993年,32(7),1531-1533)。用Wilson,NRTL和UNIQUAC方程对数据进行回归。给出了温度和气相组成的偏差,结果表明,对于所研究的两个系统,温度和气相组成的平均绝对偏差分别小于0.5%和1.1%。此外,发现在压力为16 kPa时,预测UNIFAC方程的性能要好于101.3 kPa。



























 京公网安备 11010802027423号
京公网安备 11010802027423号