当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral α‐Amino Acid‐Based NMR Solvating Agents
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-07-24 , DOI: 10.1002/hlca.202000081 Anikó Nemes 1 , Tamás Csóka 1 , Szabolcs Béni 2 , Zsófia Garádi 2 , Dénes Szabó 1 , József Rábai 1
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-07-24 , DOI: 10.1002/hlca.202000081 Anikó Nemes 1 , Tamás Csóka 1 , Szabolcs Béni 2 , Zsófia Garádi 2 , Dénes Szabó 1 , József Rábai 1
Affiliation
|
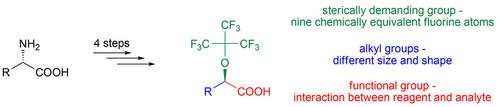
Four new chiral α‐(nonafluoro‐tert‐butoxy)carboxylic acids were synthesized from naturally occurring α‐amino acids (alanine, valine, leucine and isoleucine, respectively), and tested in 1H‐ and 19F‐NMR experiments as chiral NMR shift reagents. The NMR studies were carried out at room temperature, using CDCl3 and C6D6 as solvents, and (RS)‐α‐phenylethylamine and (RS)‐α‐(1‐naphthyl)ethylamine as racemic model compounds. To demonstrate the applicability of the reagents, the racemic drugs ketamine and prasugrel were also tested.
中文翻译:

基于手性α氨基酸的核磁共振溶剂
从天然存在的α-氨基酸(分别为丙氨酸,缬氨酸,亮氨酸和异亮氨酸)合成了四种新的手性α-(九氟叔丁氧基)羧酸,并在1 H -NMR和19 F-NMR实验中进行了手性NMR测试转移试剂。NMR研究是在室温下进行的,使用CDCl 3和C 6 D 6作为溶剂,并使用(RS)-α-苯乙胺和(RS)-α-(1-萘基)乙胺作为外消旋模型化合物。为了证明该试剂的适用性,还测试了外消旋药物氯胺酮和普拉格雷。
更新日期:2020-07-24
中文翻译:

基于手性α氨基酸的核磁共振溶剂
从天然存在的α-氨基酸(分别为丙氨酸,缬氨酸,亮氨酸和异亮氨酸)合成了四种新的手性α-(九氟叔丁氧基)羧酸,并在1 H -NMR和19 F-NMR实验中进行了手性NMR测试转移试剂。NMR研究是在室温下进行的,使用CDCl 3和C 6 D 6作为溶剂,并使用(RS)-α-苯乙胺和(RS)-α-(1-萘基)乙胺作为外消旋模型化合物。为了证明该试剂的适用性,还测试了外消旋药物氯胺酮和普拉格雷。


























 京公网安备 11010802027423号
京公网安备 11010802027423号