当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
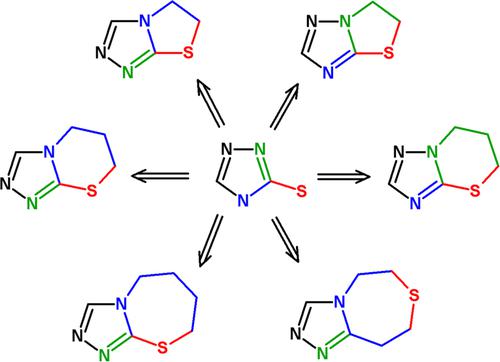
Fused bicyclic 1,2,4‐triazoles with one extra sulfur atom: Synthesis, properties, and biological activity
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-02 , DOI: 10.1002/jhet.4044 Mikhailo V. Slivka 1 , Natalia I. Korol 1 , Maksym M. Fizer 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-02 , DOI: 10.1002/jhet.4044 Mikhailo V. Slivka 1 , Natalia I. Korol 1 , Maksym M. Fizer 1
Affiliation
|
Fused heterocyclic systems with 1,2,4‐triazole scaffold arouse great interest from researchers in the branch of heterocyclic and medical chemistry because of their wide biological activities as they are considered as fungicidal, antimicrobial, analgesic, bronchodilators, antioxidant, anti‐inflammatory agents, and they are G‐quadruple stabilizers; the molecular docking data for coumarin‐containing 1,2,4‐triazoles indicate their ability to act as a urease inhibitor. Therefore, the present review aims to investigate new trends in the chemistry of heterocycles incorporating thiazolo[3,2‐b][1,2,4]triazoles, thiazolo[2,3‐c][1,2,4]triazoles, thiazino[5,1‐b][1,2,4]triazoles, thiazino[3,4‐b][1,2,4]triazoles, triazolothiazepines, and their biological characteristics. The main sections discuss: (a) the synthetic routes to the production of substituted fused heterocyclic systems, which include condensation reactions, multiple bond annulation, and the reactions of electrophilic heterocyclization. (b) Description of chemical and biological characteristics of these fused heterocycles.
中文翻译:

具有一个额外硫原子的稠合双环1,2,4-三唑:合成,性质和生物活性
具有1,2,4-三唑支架的熔融杂环系统因其广泛的生物学活性而引起杂环和医学化学领域的研究人员的极大兴趣,因为它们被认为是杀真菌,抗菌,止痛,支气管扩张剂,抗氧化剂,抗炎剂,它们是G四倍稳定剂;含有香豆素的1,2,4-三唑的分子对接数据表明它们具有充当脲酶抑制剂的能力。因此,本综述旨在研究掺入噻唑[3,2-b] [1,2,4]三唑,噻唑[2,3-c] [1,2,4]三唑的杂环化学新趋势,噻嗪基[5,1–b] [1,2,4]三唑,噻嗪基[3,4–b] [1,2,4]三唑,三唑并噻唑烷及其生物学特性。主要部分讨论:(a)生产取代的稠合杂环系统的合成途径,包括缩合反应,多键环化和亲电杂环化反应。(b)描述这些稠合杂环的化学和生物学特性。
更新日期:2020-09-08
中文翻译:

具有一个额外硫原子的稠合双环1,2,4-三唑:合成,性质和生物活性
具有1,2,4-三唑支架的熔融杂环系统因其广泛的生物学活性而引起杂环和医学化学领域的研究人员的极大兴趣,因为它们被认为是杀真菌,抗菌,止痛,支气管扩张剂,抗氧化剂,抗炎剂,它们是G四倍稳定剂;含有香豆素的1,2,4-三唑的分子对接数据表明它们具有充当脲酶抑制剂的能力。因此,本综述旨在研究掺入噻唑[3,2-b] [1,2,4]三唑,噻唑[2,3-c] [1,2,4]三唑的杂环化学新趋势,噻嗪基[5,1–b] [1,2,4]三唑,噻嗪基[3,4–b] [1,2,4]三唑,三唑并噻唑烷及其生物学特性。主要部分讨论:(a)生产取代的稠合杂环系统的合成途径,包括缩合反应,多键环化和亲电杂环化反应。(b)描述这些稠合杂环的化学和生物学特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号