Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.tetlet.2020.152197 Seong-Ryu Joo , In-Kyun Lim , Seung-Hoi Kim
|
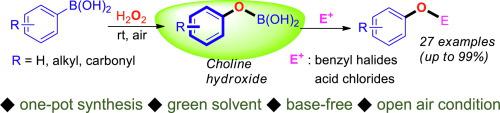
An efficient synthetic route to benzyl phenyl ether preparation has been successfully developed via a one-pot synthetic protocol utilizing a combination of arylboronic acids, hydrogen peroxide (H2O2), and benzyl halides. The whole procedure consists of two consecutive reactions, formation of boron phenoxide from arylboronic acids and its nucleophilic attack. A simple operation under mild conditions such as room-temperature ionic liquid (choline hydroxide), aerobic environment, and absence of metal- and base-catalysts has been employed. Expansion to utilize benzyl surrogates was also successfully accomplished.
中文翻译:

由芳基硼酸制备苯酚硼的另一种方法及其在
通过使用芳基硼酸,过氧化氢(H 2 O 2)和苄基卤化物的组合的一锅合成方案,已经成功地开发了制备苄基苯基醚的有效合成途径。整个过程包括两个连续的反应,由芳基硼酸形成苯氧硼及其亲核攻击。已经采用了在温和条件下的简单操作,例如室温离子液体(氢氧化胆碱),有氧环境以及不存在金属和碱催化剂。也成功地完成了利用替代代苯甲酸苄酯的扩展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号