Tetrahedron ( IF 2.1 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.tet.2020.131370 Jin Young Chai , Hyojin Cha , Hyeong Baik Kim , Dae Yoon Chi
|
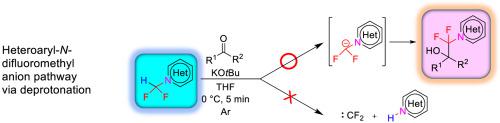
There has been great interest in the chemistry, syntheses and reactivities of heteroaryl-N-difluoromethane. The present work is based on the addition reaction of difluoromethyl anions, which are generated directly from difluoromethyl heterocycles, to benzophenone and benzaldehyde. As 1,2,3-triazoles and benzotriazoles can act as leaving groups, two reaction pathways are expected to exist: either the desired reaction route - deprotonation (formation of difluoromethyl anion) or the unfavored reaction route – the formation of difluorocarbene. We describe the chemistry for the selective addition reactions of difluoromethyltriazoles to ketones and aldehydes without the formation of difluorocarbene. Addition reactions of 1-(difluoromethyl)-1H-benzotriazole 1 to benzophenone 2 using potassium t-butoxide as a base were found to proceed smoothly at 0 °C for 5 min with high yields (80–88%). A plausible mechanism for the addition reactions of 1-(difluoromethyl)-1H-benzotriazole 1 and 1-difluoromethyl-4-phenyltriazole 5 to benzophenones and benzaldehydes is proposed based on deuterium-quenching experiments.
中文翻译:

二氟甲基三唑与酮和醛的选择性加成反应而不会形成二氟卡宾
人们对杂芳基-N-二氟甲烷的化学,合成和反应性非常感兴趣。本工作基于直接由二氟甲基杂环生成的二氟甲基阴离子与二苯甲酮和苯甲醛的加成反应。由于1,2,3-三唑和苯并三唑可以作为离去基团,因此预计存在两个反应途径:所需的反应途径-去质子化(二氟甲基阴离子的形成)或不利的反应途径-二氟卡宾的形成。我们描述了在不形成二氟卡宾的情况下二氟甲基三唑对酮和醛的选择性加成反应的化学反应。1-(二氟甲基)-1 H-苯并三唑1的加成反应以叔丁醇钾为碱制备二苯甲酮2的过程,发现在0°C下平稳进行5分钟,产率高(80-88%)。基于氘化猝灭实验,提出了一种可能的机理,即1-(二氟甲基)-1 H-苯并三唑1和1-二氟甲基-4-苯基三唑5与二苯甲酮和苯甲醛的加成反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号