Materials Today Energy ( IF 9.3 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.mtener.2020.100462 Lin Lv , Yaoxing Chang , Xiang Ao , Zhishan Li , Jian-Gang Li , Ying Wu , Xinying Xue , Yulin Cao , Guo Hong , Chundong Wang
|
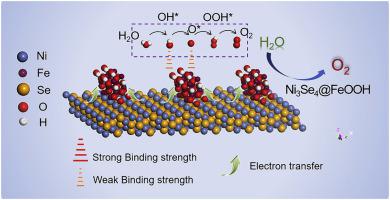
Exploring high-active catalyst for oxygen evolution reaction (OER) is paramount for efficient and eco-friendly conversion of renewable electricity to hydrogen fuels. In this study, we report a new strategy to significantly enhance the OER activity of Ni3Se4 nanochains via growth of uniformly vertical FeOOH ultrathin nanoneedles on the surface. The as-prepared catalyst demonstrates splendid OER performance with a low overpotential of 249.0 mV for driving a current density of 10 mA cm−2, yielding a small Tafel slope of 46 mV dec−1. After continuous operation of 10 h, a tiny degeneration of 4.0% is afforded, evidencing the excellent durability of the catalyst. On the basis of electrochemical measurement together with theoretical analysis, we attribute the boosted OER kinetics to the synergistic effect of electron, geometry and interface, concretely, two main reasons should be emphasized: 1) the complementary adsorption/desorption nature of nickel and iron leads to optimized Gibbs free energy; 2) redistribution of localized π-symmetry electrons at the interface endows the favorable adsorption/desorption for the oxygenated species. We anticipate that our work would push boundaries for the fabrication of high-performance transition metal- and selenium-based electrocatalysts.
中文翻译:

Ni 3 Se 4 / FeOOH上异质结构上的界面电子转移可在碱性溶液中实现高效水氧化
探索用于氧气析出反应(OER)的高活性催化剂对于将可再生电力高效且环保地转化为氢燃料至关重要。在这项研究中,我们报告了一种通过在表面均匀生长垂直的FeOOH超薄纳米针来显着增强Ni 3 Se 4纳米链的OER活性的新策略。所制备的催化剂显示出出色的OER性能,驱动电流密度为10 mA cm -2时具有249.0 mV的低过电势,产生46 mV dec -1的小Tafel斜率。连续运行10小时后,产生了4.0%的微小变性,证明了催化剂的优异耐久性。在电化学测量和理论分析的基础上,我们将增强的OER动力学归因于电子,几何形状和界面的协同效应,具体应强调两个主要原因:1)镍和铁铅的互补吸附/解吸性质优化吉布斯自由能;2)界面上局部π对称电子的重新分布赋予了含氧物质良好的吸附/解吸作用。我们期望我们的工作将突破高性能过渡金属和硒基电催化剂的制造界限。



























 京公网安备 11010802027423号
京公网安备 11010802027423号