Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapor–liquid equilibrium at 94 kPa and surface tension at 298.15 K for hexane + ethanol + cyclopentyl methyl ether mixture
Fuel ( IF 7.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fuel.2020.118415 Gustavo Chaparro , Marcela Cartes , Andrés Mejía
Fuel ( IF 7.4 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.fuel.2020.118415 Gustavo Chaparro , Marcela Cartes , Andrés Mejía
|
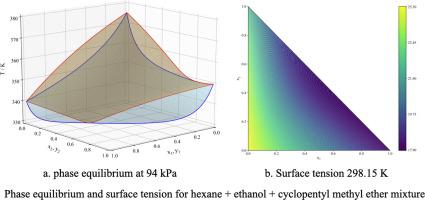
Abstract Vapor-liquid equilibrium (VLE) and surface tension (ST) for the hexane + ethanol + cyclopentyl methyl ether mixture have been measured and modeled. VLE determinations are carried out in a dynamic Guillespie type cell at the isobaric condition of 94 kPa, whereas the dependence of ST on concentration is measured in a maximum differential bubble pressure tensiometer at atmospheric pressure and 298.15 K. The thermodynamical consistent VLE data exhibit positive deviation from ideal behavior without ternary azeotropy and are well correlated by Redlich–Kister expansion and predicted by the binary nonrandom two-liquid, Wilson and universal quasichemical activity coefficient models. The ST data exhibit negative deviation from the linear behavior and are smoothed using the Myers-Scott expansion, showing no ternary aneotropic behavior. The experimental data of VLE and ST are accurately characterized by applying the square gradient theory to the Peng–Robinson Stryjek–Vera equation of state (EoS) appropriately extended to mixtures employing the modified Huron–Vidal mixing rule. This theoretical framework shows that experimental VLE and ST data can be fully predicted by only using binary contributions within a global average absolute deviation of 1.25% for VLE and 6.1% for ST. The theoretical approach also provides a route to explore the concentration distribution of species in the interfacial region, where it is possible to conclude that hexane exhibits both adsorption and desorption; ethanol displays strong positive adsorption whereas CPME does not exhibit surface activity.
中文翻译:

己烷 + 乙醇 + 环戊基甲基醚混合物的汽液平衡在 94 kPa 和表面张力在 298.15 K
摘要 已测量并模拟了己烷 + 乙醇 + 环戊基甲基醚混合物的汽液平衡 (VLE) 和表面张力 (ST)。VLE 测定是在 94 kPa 等压条件下的动态 Guillespie 型池中进行的,而 ST 对浓度的依赖性是在大气压和 298.15 K 下在最大气泡差压张力计中测量的。热力学一致的 VLE 数据表现出正偏差来自没有三元共沸的理想行为,并且通过 Redlich-Kister 膨胀与二元非随机两液体、威尔逊和通用准化学活度系数模型预测良好相关。ST 数据表现出与线性行为的负偏差,并使用 Myers-Scott 展开进行平滑,显示没有三元各向异性行为。VLE 和 ST 的实验数据通过将平方梯度理论应用于 Peng-Robinson Stryjek-Vera 状态方程 (EoS) 来准确表征,该方程适当扩展到采用改进的 Huron-Vidal 混合规则的混合物。该理论框架表明,仅在 VLE 的 1.25% 和 ST 的 6.1% 的全球平均绝对偏差内使用二进制贡献,就可以完全预测实验性 VLE 和 ST 数据。理论方法还提供了探索界面区域中物质浓度分布的途径,可以得出结论,己烷同时表现出吸附和解吸;乙醇表现出强烈的正吸附,而 CPME 没有表现出表面活性。
更新日期:2020-11-01
中文翻译:

己烷 + 乙醇 + 环戊基甲基醚混合物的汽液平衡在 94 kPa 和表面张力在 298.15 K
摘要 已测量并模拟了己烷 + 乙醇 + 环戊基甲基醚混合物的汽液平衡 (VLE) 和表面张力 (ST)。VLE 测定是在 94 kPa 等压条件下的动态 Guillespie 型池中进行的,而 ST 对浓度的依赖性是在大气压和 298.15 K 下在最大气泡差压张力计中测量的。热力学一致的 VLE 数据表现出正偏差来自没有三元共沸的理想行为,并且通过 Redlich-Kister 膨胀与二元非随机两液体、威尔逊和通用准化学活度系数模型预测良好相关。ST 数据表现出与线性行为的负偏差,并使用 Myers-Scott 展开进行平滑,显示没有三元各向异性行为。VLE 和 ST 的实验数据通过将平方梯度理论应用于 Peng-Robinson Stryjek-Vera 状态方程 (EoS) 来准确表征,该方程适当扩展到采用改进的 Huron-Vidal 混合规则的混合物。该理论框架表明,仅在 VLE 的 1.25% 和 ST 的 6.1% 的全球平均绝对偏差内使用二进制贡献,就可以完全预测实验性 VLE 和 ST 数据。理论方法还提供了探索界面区域中物质浓度分布的途径,可以得出结论,己烷同时表现出吸附和解吸;乙醇表现出强烈的正吸附,而 CPME 没有表现出表面活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号