Cell Calcium ( IF 4 ) Pub Date : 2020-07-03 , DOI: 10.1016/j.ceca.2020.102251 Sabrina Leverrier-Penna 1 , Olivier Destaing 2 , Aubin Penna 1
|
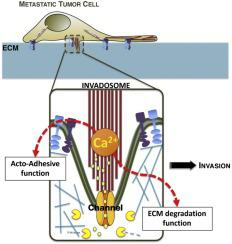
Development of metastasis causes the most serious clinical consequences of cancer and is responsible for over 90 % of cancer-related deaths. Hence, a better understanding of the mechanisms that drive metastasis formation appears critical for drug development designed to prevent the spread of cancer and related mortality. Metastasis dissemination is a multistep process supported by the increased motility and invasiveness capacities of tumor cells. To succeed in overcoming the mechanical constraints imposed by the basement membrane and surrounding tissues, cancer cells reorganize their focal adhesions or extend acto-adhesive cellular protrusions, called invadosomes, that can both contact the extracellular matrix and tune its degradation through metalloprotease activity. Over the last decade, accumulating evidence has demonstrated that altered Ca2+ channel activities and/or expression promote tumor cell-specific phenotypic changes, such as exacerbated migration and invasion capacities, leading to metastasis formation. While several studies have addressed the molecular basis of Ca2+ channel-dependent cancer cell migration, we are still far from having a comprehensive vision of the Ca2+ channel-regulated mechanisms of migration/invasion. This is especially true regarding the specific context of invadosome-driven invasion. This review aims to provide an overview of the current evidence supporting a central role for Ca2+ channel-dependent signaling in the regulation of these dynamic degradative structures. It will present available data on the few Ca2+ channels that have been studied in that specific context and discuss some potential interesting actors that have not been fully explored yet.
中文翻译:

癌性太空入侵者驾驶舱中钙通道功能的见解和观点。
转移的发展会导致癌症最严重的临床后果,并导致超过 90% 的癌症相关死亡。因此,更好地了解驱动转移形成的机制对于旨在防止癌症扩散和相关死亡率的药物开发至关重要。转移扩散是一个多步骤过程,由肿瘤细胞增加的运动性和侵袭能力支持。为了成功克服基底膜和周围组织施加的机械限制,癌细胞会重新组织它们的粘着斑或扩展称为侵袭体的活性粘附细胞突起,它们既可以接触细胞外基质,又可以通过金属蛋白酶活性调节其降解。在过去的十年中,越来越多的证据表明,改变 Ca2+通道活性和/或表达促进肿瘤细胞特异性表型变化,例如加剧的迁移和侵袭能力,导致转移形成。虽然一些研究已经解决了 Ca 2+通道依赖性癌细胞迁移的分子基础,但我们还远未对 Ca 2+通道调节的迁移/侵袭机制有一个全面的了解。对于侵入体驱动的入侵的特定背景,尤其如此。本综述旨在概述目前支持 Ca 2+通道依赖性信号传导在这些动态降解结构的调节中的核心作用的证据。它将提供关于少数 Ca 2+ 的可用数据 在特定背景下研究的渠道,并讨论一些尚未完全探索的潜在有趣参与者。


























 京公网安备 11010802027423号
京公网安备 11010802027423号