当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the development of a nucleophilic methylthiolation methodology.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-07-02 , DOI: 10.1039/d0ob01149e Bernardo Basbaum Portinho de Puga Carvalho 1 , Adriane Antonia Pereira Amaral 1 , Pedro Pôssa de Castro 1 , Fernanda Cerqueira Moreira Ferreira 1 , Bruno Araújo Cautiero Horta 2 , Giovanni Wilson Amarante 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-07-02 , DOI: 10.1039/d0ob01149e Bernardo Basbaum Portinho de Puga Carvalho 1 , Adriane Antonia Pereira Amaral 1 , Pedro Pôssa de Castro 1 , Fernanda Cerqueira Moreira Ferreira 1 , Bruno Araújo Cautiero Horta 2 , Giovanni Wilson Amarante 1
Affiliation
|
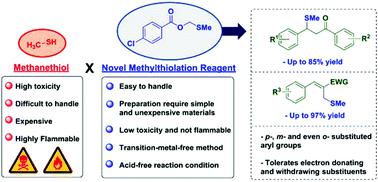
Methylthiolation reactions are usually explored to access organosulfur compounds using methanethiol, an extremely flammable and toxic compound. Herein, methylthiomethyl esters were successfully applied as novel methylthiolation reagents in a low cost, transition-metal-free methodology. These reagents allowed the methylthiolation of a wide scope of chalcones, acyl ester derivatives and Morita–Baylis–Hillman acetates with good group tolerance, affording the methylthiolated products in moderate to excellent yields. The reaction mechanism was investigated through several control experiments, as well as by theoretical calculations employing Density Functional Theory. The results strongly support that a sulfurane and a sulfonium ylide appear as key intermediates and that a Pummerer type rearrangement is also crucial for the formation of this novel reagent. Furthermore, the methylthiolation mechanism is likely to proceed through the nucleophilic attack of the reagent, followed by an entropically favoured step involving the acetate attack to the positively charged species, then releasing the product.
中文翻译:

关于亲核甲基硫醇化方法的发展。
通常使用甲硫醇(一种极易燃且有毒的化合物)来探索甲硫醇化反应,以获取有机硫化合物。在此,以低成本,无过渡金属的方法成功地将甲硫基甲基酯用作新型甲硫基化试剂。这些试剂可以对各种查尔酮,酰基酯衍生物和Morita–Baylis–Hillman乙酸盐进行甲基硫醇化,具有良好的基团耐受性,从而可以提供中等至优异收率的甲基硫醇化产品。通过一些控制实验以及采用密度泛函理论的理论计算,研究了反应机理。结果强烈支持了以硫烷和叶立德作为关键中间体出现,并且Pummerer型重排对于这种新型试剂的形成也至关重要。此外,甲硫醇化机理很可能是通过试剂的亲核攻击而进行的,随后是一个有利于熵的步骤,其中包括乙酸盐攻击带正电荷的物种,然后释放出产物。
更新日期:2020-07-22
中文翻译:

关于亲核甲基硫醇化方法的发展。
通常使用甲硫醇(一种极易燃且有毒的化合物)来探索甲硫醇化反应,以获取有机硫化合物。在此,以低成本,无过渡金属的方法成功地将甲硫基甲基酯用作新型甲硫基化试剂。这些试剂可以对各种查尔酮,酰基酯衍生物和Morita–Baylis–Hillman乙酸盐进行甲基硫醇化,具有良好的基团耐受性,从而可以提供中等至优异收率的甲基硫醇化产品。通过一些控制实验以及采用密度泛函理论的理论计算,研究了反应机理。结果强烈支持了以硫烷和叶立德作为关键中间体出现,并且Pummerer型重排对于这种新型试剂的形成也至关重要。此外,甲硫醇化机理很可能是通过试剂的亲核攻击而进行的,随后是一个有利于熵的步骤,其中包括乙酸盐攻击带正电荷的物种,然后释放出产物。

























 京公网安备 11010802027423号
京公网安备 11010802027423号