当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
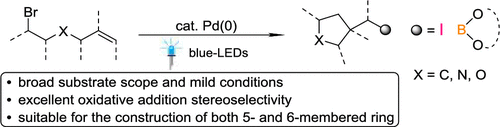
Visible-Light-Induced Palladium-Catalyzed Carbocyclization of Unactivated Alkyl Bromides with Alkenes Involving C-I or C-B Coupling.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.joc.0c00077 Jun-Wei Ma 1 , Xi Chen 1 , Zhao-Zhao Zhou 1 , Yong-Min Liang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.joc.0c00077 Jun-Wei Ma 1 , Xi Chen 1 , Zhao-Zhao Zhou 1 , Yong-Min Liang 1
Affiliation
|
A palladium-catalyzed, photochemical tandem cyclization/dicarbofunctionalization of unactivated alkyl halides containing an alkene moiety offers an appealing route to produce five- or six-membered rings in a redox-neutral fashion. Multisubstituted carbo- and heterocyclic compounds were prepared through the formation of new C–B or C–O bonds, which provides a convenient synthetic route for further transformations. This protocol is characterized by the reaction of alkene regio- and stereoselectivities, good functional group compatibility, wide substrate scope, and mild reaction conditions.
中文翻译:

可见光诱导钯催化未活化烷基溴化物与涉及CI或CB偶联的烯烃的碳环化。
钯催化的含烯烃部分的未活化烷基卤化物的光化学串联环化/双官能化提供了一条吸引人的途径,可以以氧化还原中性的方式产生五元或六元环。通过形成新的C–B或C–O键来制备多取代的碳和杂环化合物,这为进一步的转化提供了方便的合成途径。该方案的特征是烯烃的区域选择性和立体选择性反应,良好的官能团相容性,较宽的底物范围和温和的反应条件。
更新日期:2020-07-17
中文翻译:

可见光诱导钯催化未活化烷基溴化物与涉及CI或CB偶联的烯烃的碳环化。
钯催化的含烯烃部分的未活化烷基卤化物的光化学串联环化/双官能化提供了一条吸引人的途径,可以以氧化还原中性的方式产生五元或六元环。通过形成新的C–B或C–O键来制备多取代的碳和杂环化合物,这为进一步的转化提供了方便的合成途径。该方案的特征是烯烃的区域选择性和立体选择性反应,良好的官能团相容性,较宽的底物范围和温和的反应条件。


























 京公网安备 11010802027423号
京公网安备 11010802027423号