当前位置:
X-MOL 学术
›
J. Appl. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multienzymatic immobilization of laccases on polymeric microspheres: A strategy to expand the maximum catalytic efficiency
Journal of Applied Polymer Science ( IF 3 ) Pub Date : 2020-07-01 , DOI: 10.1002/app.49562 Myleidi Vera 1 , Csaba Fodor 2 , Yadiris Garcia 3 , Eduardo Pereira 3 , Katja Loos 2 , Bernabé L. Rivas 1
Journal of Applied Polymer Science ( IF 3 ) Pub Date : 2020-07-01 , DOI: 10.1002/app.49562 Myleidi Vera 1 , Csaba Fodor 2 , Yadiris Garcia 3 , Eduardo Pereira 3 , Katja Loos 2 , Bernabé L. Rivas 1
Affiliation
|
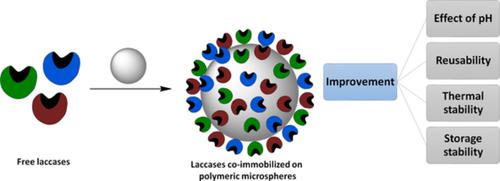
Laccase enzymes of were covalently coimmobilized on poly(glycidyl methacrylate) microspheres. The objective of this work was to create a biocatalyst that works efficiently in a wide range of pH. The coimmobilization was performed using two different strategies to compare the most efficient. The results showed that by correctly selecting the enzymes and concentrations involved in the commobilization, it is possible to obtain a biocatalyst that works efficiently at a wide pH range (2.0–7.0). The maximum activity values reached per gram of support for the obtained biocatalyst were 41.90 U (pH 3.0), 40.89 U (pH 4.0), and 39.54 U (pH 6.0). Moreover, the thermal, storage, and mechanical stabilities were improved compared to the free and single‐immobilized laccases. It was concluded that enzymatic coimmobilization is an excellent alternative to obtain a robust biocatalyst that works in a wide pH range, with potential environmental and industrial applications.
中文翻译:

漆酶在聚合物微球上的多酶固定化:扩大最大催化效率的策略
漆酶被共价共固定在聚甲基丙烯酸缩水甘油酯微球上。这项工作的目的是创造一种在广泛的pH范围内都能有效工作的生物催化剂。使用两种不同的策略进行协同固定以比较最有效的策略。结果表明,通过正确选择参与动员的酶和浓度,可以获得在宽pH范围(2.0-7.0)下有效工作的生物催化剂。对于所获得的生物催化剂,每克载体达到的最大活性值为41.90U(pH 3.0),40.89U(pH 4.0)和39.54U(pH 6.0)。此外,与游离和单固定漆酶相比,其热,储存和机械稳定性均得到改善。
更新日期:2020-07-01
中文翻译:

漆酶在聚合物微球上的多酶固定化:扩大最大催化效率的策略
漆酶被共价共固定在聚甲基丙烯酸缩水甘油酯微球上。这项工作的目的是创造一种在广泛的pH范围内都能有效工作的生物催化剂。使用两种不同的策略进行协同固定以比较最有效的策略。结果表明,通过正确选择参与动员的酶和浓度,可以获得在宽pH范围(2.0-7.0)下有效工作的生物催化剂。对于所获得的生物催化剂,每克载体达到的最大活性值为41.90U(pH 3.0),40.89U(pH 4.0)和39.54U(pH 6.0)。此外,与游离和单固定漆酶相比,其热,储存和机械稳定性均得到改善。


























 京公网安备 11010802027423号
京公网安备 11010802027423号