Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.saa.2020.118667 Balasaheb D Vanjare 1 , Prasad G Mahajan 1 , Nilam C Dige 2 , Hussain Raza 2 , Mubashir Hassan 3 , Sung-Yum Seo 2 , Ki Hwan Lee 1
|
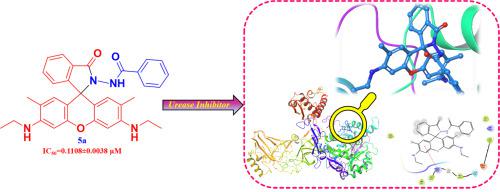
In this work, a series of the rhodamine 6G based derivatives 5a-5g, were synthesized. The structural framework of the synthesized compounds was established by using 1H NMR, 13C NMR, FT-IR, and LC-MS analytical methods. The spectroscopic properties of the target compounds were determined by using absorption and fluorescence study in four different solvents. Furthermore, the synthesized derivatives were assessed for in-vitro screening against jack bean urease inhibition and in-silico molecular docking study. The result reveals that all the compounds exhibit good urease inhibitory activity against this enzyme but among the series, the compound 5a & 5c with an IC50 values of 0.1108 ± 0.0038 μM and 0.1136 ± 0.0295 μM shows to be most auspicious inhibitory activity compared to a standard drug (Thiourea) having IC50 value 4.7201 ± 0.0546 μM. Subsequently, the molecular docking experiment was analysed to distinguish the enzyme-inhibitor binding interaction.
中文翻译:

合成新的基于x吨的类似物:其光学性质,千斤顶豆脲酶抑制作用和分子模型研究。
在这项工作中,合成了一系列基于罗丹明6G的衍生物5a - 5g。通过使用1 H NMR,13 C NMR,FT-IR和LC-MS分析方法建立了合成化合物的结构框架。通过在四种不同溶剂中的吸收和荧光研究确定目标化合物的光谱性质。此外,对合成的衍生物进行了评估,以进行针对波克豆脲酶抑制作用的体外筛选和计算机分子对接研究。结果表明,所有化合物对这种酶均表现出良好的脲酶抑制活性,但在系列中,化合物5a和5c与IC 50值为4.7201±0.0546μM的标准药物(硫脲)相比,IC 50值为0.1108±0.0038μM和0.1136±0.0295μM的化合物显示出最大的吉祥抑制活性。随后,分析了分子对接实验以区分酶-抑制剂结合相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号