Separation and Purification Technology ( IF 8.6 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.seppur.2020.117321 Bingqing Wang , Tao Fu , Baohua An , Yong Liu
|
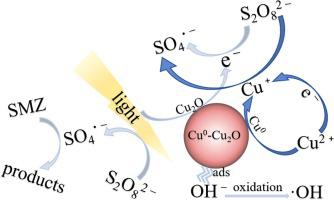
Sulfate radicals-based advanced oxidation process (SRAOP) has received increasing attention in the wastewater treatment area because sulfate radicals have high redox potential. The key for SRAOP is to develop high efficient catalysts that can effectively and stably activate persulfate (PS) to produce sulfate radicals. Cu2O is a good activator of PS and photo-catalyst. However, its catalytic activity is relatively low because of the poor stability and the recombination of electron and holes. In theory, Cu0 can reduce Cu2+ to form Cu+, which can decrease the recombination of electron and holes from the Cu2O by the formation of heterojunction. In this study, Cu0-Cu2O was synthesized by a simple ball-milling method. The as-prepared Cu0-Cu2O was characterized and used to activate PS for sulfamerazine (SMZ) degradation by means of UV illumination. In the Cu0-Cu2O/PS/UV system, the removals of 100% SMZ and 30% TOC within 30 min were achieved, respectively, and the removal efficiency of SMZ decreased by only 12.2% after 5 cycles. The high efficiency and good catalytic stability of Cu0-Cu2O for the activation of PS were due to that Cu0 and photo-induced electron of Cu2O promoted the conversion of Cu2+ to Cu+. Quenching experiments demonstrated that the hydroxyl radicals and sulfate radicals contributed to SMZ degradation. However, the concentrations and fractions of radicals was affected by the initial pH of solution. Nine intermediate products were identified, and the degradation pathways were proposed, including the fission of sulfur and nitrogen bond, the oxidation of methyl group and the hydroxylation of benzene ring. The effects of initial pH, PS concentration, Cu0-Cu2O dosage and SMZ initial concentration on the degradation of SMZ were investigated. This study provided an effective catalyst that could simultaneously activate PS and utilize UV to form photo-induced electron, which could enhance the degradation and mineralization of organic pollutants.
中文翻译:

Cu 0 -Cu 2 O紫外光辅助过硫酸盐活化降解磺胺嘧啶
基于硫酸根的高级氧化工艺(SRAOP)在废水处理领域受到越来越多的关注,因为硫酸根具有很高的氧化还原电位。SRAOP的关键是开发能够有效稳定地活化过硫酸盐(PS)产生硫酸根的高效催化剂。Cu 2 O是PS和光催化剂的良好活化剂。但是,由于稳定性差以及电子和空穴的再结合,其催化活性相对较低。从理论上讲,Cu 0可以还原Cu 2+形成Cu +,通过异质结的形成可以减少电子与空穴从Cu 2 O的复合。在这项研究中,Cu 0 -Cu 2O通过简单的球磨法合成。对所制备的Cu 0 -Cu 2 O进行表征,并通过紫外线照射将其活化以降解磺胺嘧啶(SMZ)。在Cu 0 -Cu 2 O / PS / UV系统中,分别在30分钟内实现了100%SMZ和30%TOC的去除,并且5个循环后,SMZ的去除效率仅降低了12.2%。Cu 0 -Cu 2 O对PS的活化具有很高的效率和良好的催化稳定性,这是由于Cu 0和光感应电子Cu 2 O促进了Cu 2+向Cu +的转化。。淬火实验表明,羟基自由基和硫酸根自由基有助于SMZ降解。但是,自由基的浓度和分数受溶液初始pH的影响。鉴定了九种中间产物,并提出了降解途径,包括硫和氮键的裂变,甲基的氧化和苯环的羟基化。研究了初始pH,PS浓度,Cu 0 -Cu 2 O用量和SMZ初始浓度对SMZ降解的影响。这项研究提供了一种有效的催化剂,该催化剂可以同时激活PS并利用紫外线形成光感应电子,从而可以增强有机污染物的降解和矿化作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号