European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.ejmech.2020.112561 Hanna Laaroussi 1 , Ying Ding 2 , Yuou Teng 2 , Patrick Deschamps 3 , Michel Vidal 4 , Peng Yu 2 , Sylvain Broussy 1
|
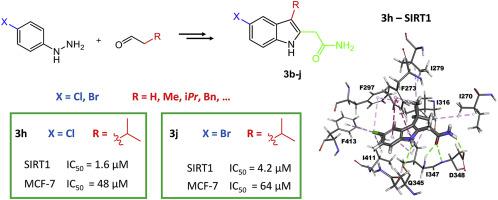
A series of achiral indole analogs of the selective sirtuin inhibitor EX-527 (a racemic, substituted 1,2,3,4 tetrahydrocarbazole) was designed to stabilize the bioactive conformation, and synthesized. These new indoles were evaluated against the isolated sirtuin enzymes SIRT1 and SIRT2, and against a panel of nine human cell lines. Structure-activity relationship studies demonstrated the influence of the substituent at position 3 of the indole. The most potent SIRT1 inhibitor 3h, bearing an isopropyl substituent, was as potent as EX-527, and more selective for SIRT1 over SIRT2. Compound 3g, bearing a benzyl substituent, inhibited both sirtuins at micromolar concentration and was more cytotoxic than EX-527 on several cancer cell lines.
中文翻译:

沉默信息调节剂1(SIRT1)吲哚抑制剂的合成及其作为细胞毒剂的评估。
设计了一系列选择性Sirtuin抑制剂EX-527的非手性吲哚类似物(外消旋,取代的1,2,3,4四氢咔唑)以稳定生物活性构象,并进行合成。针对分离的瑟土因酶SIRT1和SIRT2,以及一组九种人类细胞系,对这些新吲哚进行了评估。结构活性关系研究表明,取代基对吲哚3位的影响。带有异丙基取代基的最有效的SIRT1抑制剂3h与EX-527一样有效,并且对SIRT1的选择性高于SIRT2。在一些癌细胞系上,带有苄基取代基的化合物3g在微摩尔浓度下抑制了两种沉默调节蛋白,并且比EX-527具有更高的细胞毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号