European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-07-02 , DOI: 10.1016/j.ejmech.2020.112603 Kalpana Tilekar 1 , Neha Upadhyay 1 , Jessica D Hess 2 , Lucasantiago Henze Macias 2 , Piotr Mrowka 3 , Renato J Aguilera 2 , Franz-Josef Meyer-Almes 4 , Cristina V Iancu 5 , Jun-Yong Choe 6 , C S Ramaa 1
|
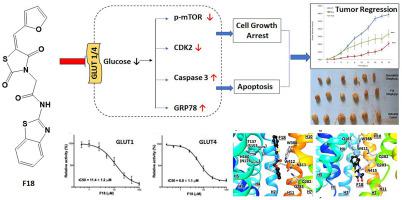
Cancer cells increase their glucose uptake and glycolytic activity to meet the high energy requirements of proliferation. Glucose transporters (GLUTs), which facilitate the transport of glucose and related hexoses across the cell membrane, play a vital role in tumor cell survival and are overexpressed in various cancers. GLUT1, the most overexpressed GLUT in many cancers, is emerging as a promising anti-cancer target. To develop GLUT1 inhibitors, we rationally designed, synthesized, structurally characterized, and biologically evaluated in-vitro and in-vivo a novel series of furyl-2-methylene thiazolidinediones (TZDs). Among 25 TZDs tested, F18 and F19 inhibited GLUT1 most potently (IC50 11.4 and 14.7 μM, respectively). F18 was equally selective for GLUT4 (IC50 6.8 μM), while F19 was specific for GLUT1 (IC50 152 μM in GLUT4). In-silico ligand docking studies showed that F18 interacted with conserved residues in GLUT1 and GLUT4, while F19 had slightly different interactions with the transporters. In in-vitro antiproliferative screening of leukemic/lymphoid cells, F18 was most lethal to CEM cells (CC50 of 1.7 μM). Flow cytometry analysis indicated that F18 arrested cell cycle growth in the subG0-G1 phase and lead to cell death due to necrosis and apoptosis. Western blot analysis exhibited alterations in cell signaling proteins, consistent with cell growth arrest and death. In-vivo xenograft study in a CEM model showed that F18 impaired tumor growth significantly.
中文翻译:

作为 GLUT 1 和 GLUT 4 抑制剂的呋喃基噻唑烷二酮衍生物的结构导向设计和合成,及其抗白血病潜力的评估。
癌细胞增加其葡萄糖摄取和糖酵解活性以满足增殖的高能量需求。葡萄糖转运蛋白 (GLUT) 促进葡萄糖和相关己糖跨细胞膜的转运,在肿瘤细胞存活中起着至关重要的作用,并在各种癌症中过度表达。GLUT1 是许多癌症中过表达最多的 GLUT,正在成为有希望的抗癌靶点。为了开发 GLUT1 抑制剂,我们合理地设计、合成、结构表征和体外和体内生物学评估了一系列新型呋喃基-2-亚甲基噻唑烷二酮 (TZD)。在测试的 25 个 TZD 中,F18 和 F19 最有效地抑制 GLUT1(IC 50分别为 11.4 和 14.7 μM)。F18 对 GLUT4 的选择性相同(IC50 6.8 μM),而 F19 对 GLUT1 具有特异性(GLUT4 中的IC 50 152 μM)。计算机配体对接研究表明,F18 与 GLUT1 和 GLUT4 中的保守残基相互作用,而 F19 与转运蛋白的相互作用略有不同。在白血病/淋巴样细胞的体外抗增殖筛选中,F18 对 CEM 细胞的杀伤力最强(CC 50为 1.7 μM)。流式细胞术分析表明,F18 在 subG0-G1 期阻滞细胞周期生长并导致细胞因坏死和凋亡而死亡。蛋白质印迹分析显示细胞信号蛋白的改变,与细胞生长停滞和死亡一致。体内 CEM 模型中的异种移植研究表明 F18 显着损害了肿瘤的生长。


























 京公网安备 11010802027423号
京公网安备 11010802027423号