当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Mechanism of K and Ca on the Volatile–Biochar Interaction for Rapid Pyrolysis of Biomass: Experimental and Simulation Studies
Energy & Fuels ( IF 5.3 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.energyfuels.0c01946 Dongdong Feng 1, 2 , Hongliang Sun 2 , Yan Ma 3 , Shaozeng Sun 2 , Yijun Zhao 2 , Dawei Guo 2 , Guozhang Chang 1 , Xiaoyong Lai 1 , Jiangquan Wu 2 , Heping Tan 2
Energy & Fuels ( IF 5.3 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.energyfuels.0c01946 Dongdong Feng 1, 2 , Hongliang Sun 2 , Yan Ma 3 , Shaozeng Sun 2 , Yijun Zhao 2 , Dawei Guo 2 , Guozhang Chang 1 , Xiaoyong Lai 1 , Jiangquan Wu 2 , Heping Tan 2
Affiliation
|
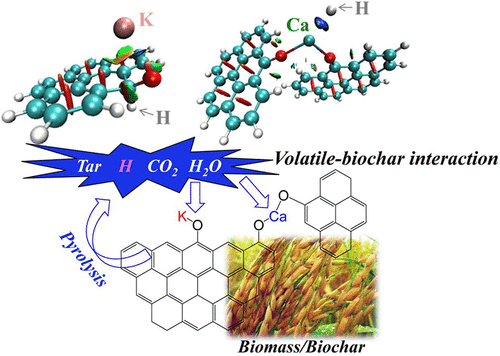
A good understanding of the catalytic mechanism of alkali and alkaline earth metal species during pyrolysis is required for the development of biomass utilization, to take advantage of its special thermochemical properties. The mechanism of the volatile–biochar interaction, especially between H radical/CO2/H2O and K/Ca in biochar, was systematically investigated by combining experimental analyses and density functional theory calculation. The results indicate that, at 500–900 °C, the yield of biochar from rice husk pyrolysis is basically constant, which mainly shows the mutual conversion between gas and liquid products. With the increase of loading alkali and alkaline earth metal species (AAEMs), the tar yield gradually decreased, and with it reaching a certain content, a significant catalytic effect of AAEMs can be observed. K is more beneficial to maintain the stability of the biochar structure than Ca. The intensities of C═O and C–O–C functional groups would appear with a certain increase with the increasing concentration of bond-linking AAEMs. At 700–900 °C, the precipitation ratio of K is almost twice that of Ca and even more. The presence of AAEMs can largely inhibit the formation of three-ring tar components, and more converted to one-ring tar components. The H radicals in the volatile can be bonded to C in the C–O–K structure, thereby causing the K element to decouple with the functional group, and tend to be adsorbed on the carbon matrix. For the Ca-loaded structure, it is manifested in strong adsorption of H radicals by Ca, resulting in the weakening of C–O bonds and the looseness of the biochar structure. The interaction between K/Ca in biochar and H2O/CO2 is reflected in the mutual attraction of AAEMs and the O atom in the H2O/CO2 molecule.
中文翻译:

钾和钙对挥发性生物炭相互作用快速生物质热解的催化机理:实验与模拟研究
为了利用生物质的特殊热化学性质,需要对热解过程中碱金属和碱土金属物种的催化机理有充分的了解,以开发生物质。挥发物-生物炭相互作用的机制,尤其是H自由基/ CO 2 / H 2之间的相互作用通过结合实验分析和密度泛函理论计算,系统地研究了生物炭中的O和K / Ca。结果表明,在500-900°C时,稻壳热解过程中生物炭的收率基本恒定,这主要表明了气态和液态产物之间的相互转化。随着碱金属和碱土金属物种(AAEMs)含量的增加,焦油收率逐渐降低,并达到一定的含量,可以观察到AAEMs的显着催化作用。与Ca相比,K对于保持生物炭结构的稳定性更有利。随着键联AAEMs浓度的增加,C═O和C–O–C官能团的强度会出现一定的增加。在700-900°C时,钾的沉淀率几乎是钙的两倍,甚至更多。AAEM的存在可以在很大程度上抑制三环焦油成分的形成,并且更多地转化为一环焦油成分。挥发物中的H自由基可以键合在C–O–K结构中的C上,从而使K元素与官能团解偶联,并倾向于吸附在碳基质上。对于负载钙的结构,它表现为钙对H自由基的强烈吸附,导致C–O键的减弱和生物炭结构的疏松。生物炭中的K / Ca与H之间的相互作用 对于负载钙的结构,它表现为钙对H自由基的强烈吸附,导致C–O键的减弱和生物炭结构的疏松。生物炭中的K / Ca与H之间的相互作用 对于负载钙的结构,它表现为钙对H自由基的强烈吸附,导致C–O键的减弱和生物炭结构的疏松。生物炭中的K / Ca与H之间的相互作用2 O / CO 2反映在AEMEM和H 2 O / CO 2分子中的O原子的相互吸引上。
更新日期:2020-08-20
中文翻译:

钾和钙对挥发性生物炭相互作用快速生物质热解的催化机理:实验与模拟研究
为了利用生物质的特殊热化学性质,需要对热解过程中碱金属和碱土金属物种的催化机理有充分的了解,以开发生物质。挥发物-生物炭相互作用的机制,尤其是H自由基/ CO 2 / H 2之间的相互作用通过结合实验分析和密度泛函理论计算,系统地研究了生物炭中的O和K / Ca。结果表明,在500-900°C时,稻壳热解过程中生物炭的收率基本恒定,这主要表明了气态和液态产物之间的相互转化。随着碱金属和碱土金属物种(AAEMs)含量的增加,焦油收率逐渐降低,并达到一定的含量,可以观察到AAEMs的显着催化作用。与Ca相比,K对于保持生物炭结构的稳定性更有利。随着键联AAEMs浓度的增加,C═O和C–O–C官能团的强度会出现一定的增加。在700-900°C时,钾的沉淀率几乎是钙的两倍,甚至更多。AAEM的存在可以在很大程度上抑制三环焦油成分的形成,并且更多地转化为一环焦油成分。挥发物中的H自由基可以键合在C–O–K结构中的C上,从而使K元素与官能团解偶联,并倾向于吸附在碳基质上。对于负载钙的结构,它表现为钙对H自由基的强烈吸附,导致C–O键的减弱和生物炭结构的疏松。生物炭中的K / Ca与H之间的相互作用 对于负载钙的结构,它表现为钙对H自由基的强烈吸附,导致C–O键的减弱和生物炭结构的疏松。生物炭中的K / Ca与H之间的相互作用 对于负载钙的结构,它表现为钙对H自由基的强烈吸附,导致C–O键的减弱和生物炭结构的疏松。生物炭中的K / Ca与H之间的相互作用2 O / CO 2反映在AEMEM和H 2 O / CO 2分子中的O原子的相互吸引上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号