当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metabolism of MMB022 and identification of dihydrodiol formation in vitro using synthesized standards.
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/dta.2888 Shimpei Watanabe 1, 2 , Xiongyu Wu 3 , Johan Dahlen 3 , Peter Konradsson 3 , Svante Vikingsson 1, 2 , Robert Kronstrand 1, 2 , Henrik Gréen 1, 2
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/dta.2888 Shimpei Watanabe 1, 2 , Xiongyu Wu 3 , Johan Dahlen 3 , Peter Konradsson 3 , Svante Vikingsson 1, 2 , Robert Kronstrand 1, 2 , Henrik Gréen 1, 2
Affiliation
|
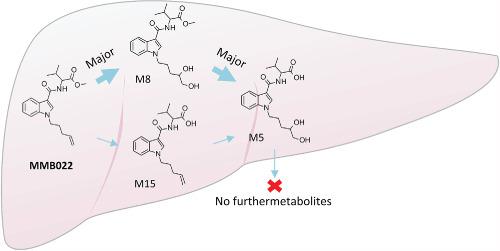
MMB022 (methyl 3‐methyl‐2‐[1‐(pent‐4‐en‐1‐yl)‐1H‐indole‐3‐carboxamido]butanoate) is a new synthetic cannabinoid with an alkene at the pentenyl side chain, a rare functional group for synthetic cannabinoids. Metabolite identification is an important step for the detection of synthetic cannabinoids in humans, since they are generally extensively metabolized. The aims of the study were to tentatively identify in vitro phase I metabolites, to confirm major metabolites using synthesized metabolites, to examine metabolic pathways thoroughly, to study metabolic stability and to suggest metabolites appropriate for urine screening. MMB022 and its synthesized metabolites were incubated with human liver microsomes (HLM) and the supernatants were analyzed by liquid chromatography‐quadrupole time‐of‐flight mass spectrometry. Sixteen metabolites were identified, which were generated via dehydrogenation, dihydrodiol formation, ester hydrolysis, hydroxylation, and combinations thereof. A major biotransformation of the alkene at the pentenyl side chain was confirmed to be dihydrodiol formation. The major metabolites were ester hydrolysis (M15) and dihydrodiol (M8) metabolites, whereas the metabolite derived from the combination of ester hydrolysis and dihydrodiol (M5) was the fourth most abundant metabolite. The metabolic pathways were investigated using synthesized metabolites and revealed that M5 is an end product of the pathways, indicating that it might become a more abundant metabolite in vivo depending on the rate of metabolism in humans. The major pathway of MMB022 to M5 was determined to be via M8 formation. Intrinsic clearance of MMB022 was determined to be 296 mL/min/kg and t1/2 was 2.1 min, indicating a low metabolic stability. M15, M8, and potentially M5 are suggested as suitable urinary targets.
中文翻译:

MMB022 的代谢和使用合成标准品在体外鉴定二氢二醇的形成。
MMB022 (甲基3-甲基-2-[1-(pent-4-en-1-yl)-1 H-indole-3-carboxamido]butanoate) 是一种新的合成大麻素,在戊烯基侧链上有一个烯烃,这是合成大麻素的罕见官能团。代谢物鉴定是检测人体合成大麻素的重要步骤,因为它们通常被广泛代谢。该研究的目的是初步鉴定体外 I 期代谢物,使用合成代谢物确认主要代谢物,彻底检查代谢途径,研究代谢稳定性并建议适合尿液筛查的代谢物。MMB022 及其合成代谢物与人肝微粒体 (HLM) 一起孵育,并通过液相色谱-四极杆飞行时间质谱法分析上清液。鉴定了 16 种代谢物,它们是通过脱氢产生的,二氢二醇形成、酯水解、羟基化及其组合。戊烯基侧链上烯烃的主要生物转化被证实是二氢二醇的形成。主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而源自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 及其组合。戊烯基侧链上烯烃的主要生物转化被证实是二氢二醇的形成。主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而源自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 及其组合。戊烯基侧链上烯烃的主要生物转化被证实是二氢二醇的形成。主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而源自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而来自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而来自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和t 1/2为2.1分钟,表明代谢稳定性低。建议 M15、M8 和潜在的 M5 作为合适的泌尿目标。
更新日期:2020-07-01
中文翻译:

MMB022 的代谢和使用合成标准品在体外鉴定二氢二醇的形成。
MMB022 (甲基3-甲基-2-[1-(pent-4-en-1-yl)-1 H-indole-3-carboxamido]butanoate) 是一种新的合成大麻素,在戊烯基侧链上有一个烯烃,这是合成大麻素的罕见官能团。代谢物鉴定是检测人体合成大麻素的重要步骤,因为它们通常被广泛代谢。该研究的目的是初步鉴定体外 I 期代谢物,使用合成代谢物确认主要代谢物,彻底检查代谢途径,研究代谢稳定性并建议适合尿液筛查的代谢物。MMB022 及其合成代谢物与人肝微粒体 (HLM) 一起孵育,并通过液相色谱-四极杆飞行时间质谱法分析上清液。鉴定了 16 种代谢物,它们是通过脱氢产生的,二氢二醇形成、酯水解、羟基化及其组合。戊烯基侧链上烯烃的主要生物转化被证实是二氢二醇的形成。主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而源自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 及其组合。戊烯基侧链上烯烃的主要生物转化被证实是二氢二醇的形成。主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而源自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 及其组合。戊烯基侧链上烯烃的主要生物转化被证实是二氢二醇的形成。主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而源自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而来自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 主要代谢物是酯水解 (M15) 和二氢二醇 (M8) 代谢物,而来自酯水解和二氢二醇 (M5) 组合的代谢物是第四大最丰富的代谢物。使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和 使用合成代谢物研究代谢途径,发现 M5 是这些途径的终产物,表明它可能成为体内更丰富的代谢物,具体取决于人体的代谢速率。MMB022 到 M5 的主要途径被确定为通过 M8 形成。MMB022 的内在清除率确定为 296 mL/min/kg 和t 1/2为2.1分钟,表明代谢稳定性低。建议 M15、M8 和潜在的 M5 作为合适的泌尿目标。


























 京公网安备 11010802027423号
京公网安备 11010802027423号