当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pd‐Catalyzed Regioselective Olefination of N‐Tosylhydrazones with Benzyl Bromides
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202002010 Zhenze Sun 1 , Jing He 1 , Weiwei Li 1 , Xuezhen Li 1 , Yijiao Feng 1 , Yan Liu 1 , Ping Liu 1 , Sheng Han 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202002010 Zhenze Sun 1 , Jing He 1 , Weiwei Li 1 , Xuezhen Li 1 , Yijiao Feng 1 , Yan Liu 1 , Ping Liu 1 , Sheng Han 1
Affiliation
|
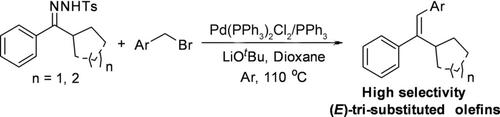
A palladium‐catalyzed regioselective olefination of cycloalkylphenylmethylene N ‐tosylhydrazones and benzyl bromide was developed. A series of E ‐type tri‐substituted olefin compounds were obtained in 43–95% through the highly selective β ‐H elimination. The results showed that the β ‐H at the benzyl position took precedence over the β ‐H at the cycloalkyl position for the alkylpalladium intermediate, avoiding the formation of tetra‐substituted olefins. High regioselectivity, wide substrate scope, and good functional group tolerance are the advantages of this reaction.
中文翻译:

钯催化N-甲苯磺酰azo与苄基溴的区域选择性烯烃化
开发了钯催化的环烷基苯基亚甲基N-甲苯磺酰基hydr和苄基溴的区域选择性烯化反应。通过高度选择性的β- H消除,可以得到43%至95%的一系列E型三取代烯烃化合物。结果表明,该β在苄基位-H了优先于β -H在该alkylpalladium中间环烷基的位置,避免了四取代烯烃的形成。高区域选择性,宽的底物范围和良好的官能团耐受性是该反应的优点。
更新日期:2020-07-01
中文翻译:

钯催化N-甲苯磺酰azo与苄基溴的区域选择性烯烃化
开发了钯催化的环烷基苯基亚甲基N-甲苯磺酰基hydr和苄基溴的区域选择性烯化反应。通过高度选择性的β- H消除,可以得到43%至95%的一系列E型三取代烯烃化合物。结果表明,该β在苄基位-H了优先于β -H在该alkylpalladium中间环烷基的位置,避免了四取代烯烃的形成。高区域选择性,宽的底物范围和良好的官能团耐受性是该反应的优点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号