Journal of Solid State Chemistry ( IF 3.3 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.jssc.2020.121459 Saida Gharouel , Youssef Ben Smida , Mounir Ferhi , Josefa Isasi Marin , Lorena Alcaraz Romo , Pablo Arévalo Cid , Karima Horchani-Naifera , Mokhtar Férid
|
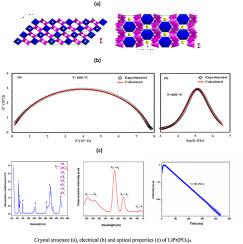
A lithium praseodymium polyphosphate LiPr(PO3)4 sample was synthesized by flux method, and its crystal structure was refined by the Rietveld method.The studied sample crystallizes in an isotopic structure with other polyphosphates, with the general formula LiLn(PO3)4 [Ln = La, Ce, Nd, Sm and Eu].Refinement results have allowed us to establish the location of the atoms in the monoclinic system of space group C2/c as follows: Li/Pr (4e) and P/O (8f); being the unit cell parameters a = 16.462(4) Å; b = 7.076(2) Å; c = 9.774(3) Å and β = 126.186(15)°. The structure of LiPr(PO3)4 sample can be best described in terms of a three-dimensional framework formed by an infinite spiral (PO3)n chains linked by PrO8 polyhedra and in which Li+ cations are in tunnels that run along the [101] direction. The structural model was supported by the charge distribution (CHARDI) and Bond Valence Sum (BVS) models. The ionic conductivity of this phosphate has also been determined by complex impedance spectroscopy measurements in pressed pellets and the obtained data suggest the unique contribution of Li+ mobile cations with activation energies of 0.49 and 1.07eV.The movement of the Li+ cations analyzed by the Bond Valence Site Energy model finds Li+ cations that move in 1D routes along [101] with an empirical activation energy of approximately 0.58 eV, value very close to that determined by impedance spectroscopy measurements. Finally, different optical parameters (absorption and emission spectra, refractive index, the band interval and color coordinates) were measured to investigate the optical properties of this polyphosphate. A persistent luminescence in the yellow-green region due to 3P0 → 3H5, 3H6, 3F2 and 3F4 transitions of Pr3+ ions, being the decay time from 3P0 level of 61.76 ns.
中文翻译:

LiPr(PO 3)4的合成,结构,电学和光学性质
采用助熔剂法合成了phosphate磷酸锂多磷酸锂LiPr(PO 3)4样品,采用Rietveld方法精制了其晶体结构,所研究的样品与其他多磷酸盐在同位素结构中结晶,通式为LiLn(PO 3)4 [Ln = La,Ce,Nd,Sm和Eu]。精炼结果使我们能够确定原子在C2 / c空间群单斜系统中的位置,如下所示:Li / Pr(4e)和P / O(8楼); 是单位晶格参数a = 16.462(4)Å; b = 7.076(2)Å; c = 9.774(3)Å和β= 126.186(15)°。LiPr(PO 3)4的结构用由PrO 8多面体连接的无限螺旋(PO 3)n链形成的三维框架可以很好地描述样品,其中Li +阳离子在沿[101]方向延伸的隧道中。结构模型由电荷分布(CHARDI)和键价和(BVS)模型支持。这种磷酸盐的离子导电性也得到了在压片复阻抗谱测量来确定所获得的数据表明Li的独特贡献+移动阳离子与0.49活化能和锂的1.07eV.The运动+阳离子由被分析债券价位能模型发现Li +沿[101]沿1D路径移动的阳离子的经验活化能约为0.58 eV,其值非常接近于通过阻抗谱测量确定的值。最后,测量了不同的光学参数(吸收和发射光谱,折射率,能带间隔和色坐标)以研究该多磷酸盐的光学性质。在黄-绿区由于持久性发光3 P 0 → 3 ħ 5,3 ħ 6,3 ˚F 2和3个˚F 4个转变的Pr 3+离子,与所述衰减时间3 P0电平为61.76 ns。


























 京公网安备 11010802027423号
京公网安备 11010802027423号