Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.bioorg.2020.104052 Rikeshwer Prasad Dewangan 1 , Shalini Kumari 2 , Aman Kumar Mahto 1 , Aditi Jain 2 , Santosh Pasha 2
|
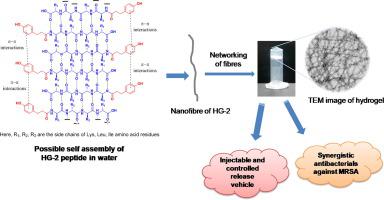
Self assembly is a ubiquitous process of complex bio-molecules to perform various biological functions. This bottom-up approach applies in engineering of various nanostructures in different technological and biomedical applications. Here we report design and synthesis of phenolic acid conjugated tetra peptides which self assembled in uniform nanofibrils upon dissolution in aqueous solutions at physiological pH and formed stiff and transparent hydrogel. Gel inversion assay, HR-TEM, FT-IR, CD spectroscopy and rheometric analysis characterized the developed hydrogel (HG-2). This gel exhibits characteristics of thixotropy and injectability. Structure-gelation relationship studies of peptide revealed the importance of π-π interactions in self assembly and hydrogelation. Further, this hydrogel used for entrapment and sustained release of antibiotics, rifampicin and ciprofloxacin at physiological pH and temperature for 5 days. The hydrogelator peptide has shown moderate antibacterial activity alone, whereas in combination with rifampicin and ciprofloxacin showed a remarkable synergistic antibacterial activity against clinically relevant multidrug resistant methicillin resistant S. aureus (MRSA). Interestingly, this hydrogel neither cause significant damage to hRBCs nor to human keratinocyte up to hydrogelation concentrations tested by haemolytic and MTT assay. These characteristics of present peptide hold future promising soft materials for treatment of infections and drug delivery applications.
中文翻译:

N末端修饰的四肽的自组装和水凝胶化,用于抗菌药物对耐甲氧西林的金黄色葡萄球菌的缓释和协同作用。
自组装是复杂的生物分子无处不在的过程,可以执行各种生物学功能。这种自下而上的方法适用于不同技术和生物医学应用中的各种纳米结构的工程设计。在这里,我们报告了酚酸共轭四肽的设计和合成,该四肽在生理pH值下溶于水溶液后会自组装成均匀的纳米原纤维,并形成坚硬透明的水凝胶。凝胶反转分析,HR-TEM,FT-IR,CD光谱和流变分析对已开发的水凝胶(HG-2)。这种凝胶具有触变性和可注射性。肽的结构-凝胶关系研究揭示了π-π相互作用在自组装和水凝胶化中的重要性。此外,该水凝胶用于在生理pH和温度下截留并持续释放抗生素,利福平和环丙沙星5天。单独的水凝胶剂肽已显示出中等的抗菌活性,而与利福平和环丙沙星合用对临床相关的耐多药性耐甲氧西林金黄色葡萄球菌具有显着的协同抗菌活性。(MRSA)。有趣的是,这种水凝胶既不会对hRBC造成明显损害,也不会对人角质形成细胞造成严重损害,直至通过溶血和MTT分析测试的水凝胶浓度。本发明肽的这些特性为治疗感染和药物输送应用提供了有前途的有希望的软材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号