Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.apcata.2020.117699 Anjaneyulu Chatla , Minhaj M. Ghouri , Omar Wissam El Hassan , Nosaiba Mohamed , Anuj V. Prakash , Nimir O. Elbashir
|
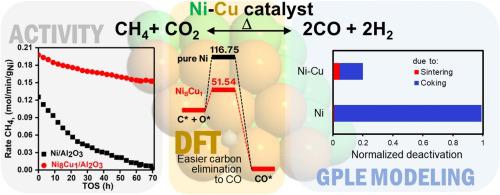
The influence of varying Cu atomic ratios in a bimetallic Ni-Cu catalytic system for the CO2 or Dry Reforming of Methane (DRM) is investigated experimentally, in conjunction with ab-initio Density Functional Theory (DFT) and Generalized Power Law Expression (GPLE)-based mathematical modeling approaches. Among the synthesized catalysts, an 8:1 Ni:Cu ratio shows highest activity with stable H2/CO selectivity compared to its monometallic counterpart. Detailed physico-chemical characterization of the catalysts indicate that copper addition minimizes deactivation by forming a Ni-Cu alloy that increases reducibility of the NiO and limits the tendency of the active site toward coke formation. DFT calculations reveal increased energy barrier for carbon adsorption, whilst promoting facile removal of deposited carbon. GPLE analysis of the deactivation profiles suggest coking as the primary deactivation route in monometallic Ni catalyst; although much lesser in extent, deactivation of Ni8Cu1/Al2O3 catalyst is induced by both sintering and coking regimes.
中文翻译:

实验和第一原理DFT研究Ni / Al 2 O 3催化剂中添加Cu对甲烷干重整的影响
结合从头算密度泛函理论(DFT)和广义幂定律表达式(GPLE),通过实验研究了双金属Ni-Cu催化体系中不同的Cu原子比对CO 2或甲烷的干重整的影响。 )的数学建模方法。在合成的催化剂中,Ni:Cu为8:1的比例显示出最高的活性和稳定的H 2/ CO选择性与其单金属对应物相比。催化剂的详细物理化学特征表明,铜的添加可通过形成Ni-Cu合金来最大程度地减少失活,该合金可提高NiO的还原性并限制活性部位形成焦炭的趋势。DFT计算显示出增加的碳吸附能垒,同时促进了沉积碳的轻松去除。GPLE对失活曲线的分析表明,在单金属Ni催化剂中,焦化是主要的失活途径。尽管程度要小得多,但烧结和焦化方式均会引起Ni 8 Cu 1 / Al 2 O 3催化剂失活。


























 京公网安备 11010802027423号
京公网安备 11010802027423号