当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, photophysical and electropolymerization properties of thiophene-substituted 2,3-diphenylbuta-1,3-dienes
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-06-30 , DOI: 10.1039/d0nj02382e Maxime Roger 1, 2, 3, 4, 5 , Kassem Amro 1, 2, 3, 4, 5 , Joëlle Rault-Berthelot 3, 6, 7, 8, 9 , Mathias Quiot 1, 2, 3, 4, 5 , Arie Van der Lee 3, 4, 10, 11, 12 , Cyril Poriel 3, 6, 7, 8, 9 , Sébastien Richeter 1, 2, 3, 4, 5 , Sébastien Clément 1, 2, 3, 4, 5 , Philippe Gerbier 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020-06-30 , DOI: 10.1039/d0nj02382e Maxime Roger 1, 2, 3, 4, 5 , Kassem Amro 1, 2, 3, 4, 5 , Joëlle Rault-Berthelot 3, 6, 7, 8, 9 , Mathias Quiot 1, 2, 3, 4, 5 , Arie Van der Lee 3, 4, 10, 11, 12 , Cyril Poriel 3, 6, 7, 8, 9 , Sébastien Richeter 1, 2, 3, 4, 5 , Sébastien Clément 1, 2, 3, 4, 5 , Philippe Gerbier 1, 2, 3, 4, 5
Affiliation
|
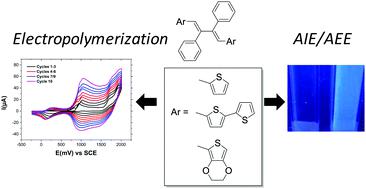
A series of 2,3-diphenylbuta-1,3-dienes (DPBs) bearing thiophene (T-DPB), bithiophene (BT-DPB) and ethylenedioxythiophene (EDOT-DPB) electropolymerizable units were prepared through desilylation reactions of the corresponding 3,4-diphenylsilole derivatives. These DPB derivatives exhibit remarkably different aggregation induced emission (AIE) or aggregation enhanced emission (AEE) behaviour depending on the strength of the molecular interactions occurring in the solid state. Indeed, T-DPB and EDOT-DPB were found to be good AIEgens while BT-DPB exhibited AEE behaviour. Finally, the electrochemical properties of these new materials were investigated revealing for all DPBs the occurrence of electropolymerization processes leading potentially to low band gap polymers.
中文翻译:

噻吩取代的2,3-二苯基丁-1,3-二烯的合成,光物理和电聚合特性
通过相应3个化合物的脱甲硅基反应,制备了一系列带有噻吩(T-DPB),联噻吩(BT-DPB)和乙二氧基噻吩(EDOT-DPB)的2,3-二苯丁1,3-二烯(DPBs), 4-二苯基甲硅烷基衍生物。这些DPB衍生物表现出明显不同的聚集诱导发射(AIE)或聚集增强发射(AEE)行为,具体取决于固态发生的分子相互作用的强度。确实,发现T-DPB和EDOT-DPB是好的AIEgens,而BT-DPB表现出AEE行为。最后,对这些新材料的电化学性能进行了研究,发现所有DPB均发生电聚合过程,从而可能导致低带隙聚合物。
更新日期:2020-07-27
中文翻译:

噻吩取代的2,3-二苯基丁-1,3-二烯的合成,光物理和电聚合特性
通过相应3个化合物的脱甲硅基反应,制备了一系列带有噻吩(T-DPB),联噻吩(BT-DPB)和乙二氧基噻吩(EDOT-DPB)的2,3-二苯丁1,3-二烯(DPBs), 4-二苯基甲硅烷基衍生物。这些DPB衍生物表现出明显不同的聚集诱导发射(AIE)或聚集增强发射(AEE)行为,具体取决于固态发生的分子相互作用的强度。确实,发现T-DPB和EDOT-DPB是好的AIEgens,而BT-DPB表现出AEE行为。最后,对这些新材料的电化学性能进行了研究,发现所有DPB均发生电聚合过程,从而可能导致低带隙聚合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号