当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights on the Behavior of Imidazolium Ionic Liquids as Electrolytes in Carbon-Based Supercapacitors: An Applied Electrochemical Approach
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jpcc.0c04217 Paulo F. R. Ortega 1 , Garbas A. dos Santos 2 , João P. C. Trigueiro 3 , Glaura G. Silva 2 , Noemí Quintanal 4 , Clara Blanco 4 , Rodrigo L. Lavall 2 , Ricardo Santamaría 4
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jpcc.0c04217 Paulo F. R. Ortega 1 , Garbas A. dos Santos 2 , João P. C. Trigueiro 3 , Glaura G. Silva 2 , Noemí Quintanal 4 , Clara Blanco 4 , Rodrigo L. Lavall 2 , Ricardo Santamaría 4
Affiliation
|
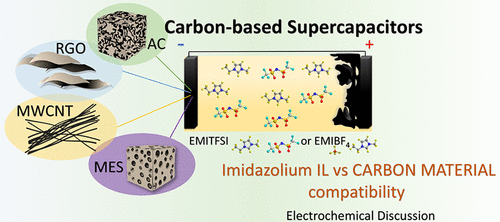
This study aims to increase the knowledge on the interactions that occur at the electrode/electrolyte interface in carbon-based electric double-layer capacitors (EDLCs) when solvent-free ionic liquids are used as electrolytes. Many previous studies found in the literature are conducted using theoretical approaches, and they are unable to model all the variables and the complexity of an actual device with a complex carbon surface and an ionic liquid (IL). Here, the compatibility between imidazolium ionic liquids and different carbon materials—an activated carbon (AC), a mesoporous carbon (MES), multiwalled carbon nanotubes (MWCNTs), and reduced graphene oxide (RGO)—is empirically investigated applying synchronous chronopotentiometric tests to various symmetrical EDLCs. The study of the simultaneous evolution of the cell and electrode potentials of the various carbon/ILs cells, monitoring the evolution of specific capacitances and electrical resistances for each independent electrode, allows inferring about the ion–electrode compatibility, the limiting factors for charge accumulation, and its impacts on the performance of the global cell. The results indicate that the sp2 structures of MWCNTs and RGO favor interactions with the EMI+ cation on the negative electrode. In the positive electrodes, MES and AC favor interactions with the BF4– and TFSI– anions, respectively, yielding a higher specific capacitance and lower resistance.
中文翻译:

咪唑鎓离子液体作为碳基超级电容器中电解质行为的见解:一种应用的电化学方法
这项研究的目的是增加有关将无溶剂离子液体用作电解质时碳基双电层电容器(EDLC)中电极/电解质界面发生相互作用的知识。文献中发现的许多以前的研究都是使用理论方法进行的,它们无法模拟具有复杂碳表面和离子液体(IL)的实际设备的所有变量和复杂性。在这里,对咪唑鎓离子液体与不同碳材料(活性炭(AC),中孔碳(MES),多壁碳纳米管(MWCNT)和氧化石墨烯(RGO))之间的相容性进行了经验性的同步同步电位测试各种对称的EDLC。对各种碳/ ILs电池的电池电位和电极电位同时演变的研究,监视每个独立电极的比电容和电阻的演变,可以推断出离子-电极的相容性,电荷积累的限制因素,及其对全球电池性能的影响。结果表明MWCNTs和RGO的2种结构有利于与负极上的EMI +阳离子相互作用。在正电极,MES和AC青睐相互作用与BF 4 -和TFSI -阴离子分别产生较高的比电容和较低的电阻。
更新日期:2020-07-23
中文翻译:

咪唑鎓离子液体作为碳基超级电容器中电解质行为的见解:一种应用的电化学方法
这项研究的目的是增加有关将无溶剂离子液体用作电解质时碳基双电层电容器(EDLC)中电极/电解质界面发生相互作用的知识。文献中发现的许多以前的研究都是使用理论方法进行的,它们无法模拟具有复杂碳表面和离子液体(IL)的实际设备的所有变量和复杂性。在这里,对咪唑鎓离子液体与不同碳材料(活性炭(AC),中孔碳(MES),多壁碳纳米管(MWCNT)和氧化石墨烯(RGO))之间的相容性进行了经验性的同步同步电位测试各种对称的EDLC。对各种碳/ ILs电池的电池电位和电极电位同时演变的研究,监视每个独立电极的比电容和电阻的演变,可以推断出离子-电极的相容性,电荷积累的限制因素,及其对全球电池性能的影响。结果表明MWCNTs和RGO的2种结构有利于与负极上的EMI +阳离子相互作用。在正电极,MES和AC青睐相互作用与BF 4 -和TFSI -阴离子分别产生较高的比电容和较低的电阻。



























 京公网安备 11010802027423号
京公网安备 11010802027423号