Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Lysyl-tRNA Synthetase on the Maturation of HIV-1 Reverse Transcriptase.
ACS Omega ( IF 4.1 ) Pub Date : 2020-06-30 , DOI: 10.1021/acsomega.0c01449 Tatiana V Ilina 1 , Ryan L Slack 1 , Michel Guerrero 1 , Rieko Ishima 1
ACS Omega ( IF 4.1 ) Pub Date : 2020-06-30 , DOI: 10.1021/acsomega.0c01449 Tatiana V Ilina 1 , Ryan L Slack 1 , Michel Guerrero 1 , Rieko Ishima 1
Affiliation
|
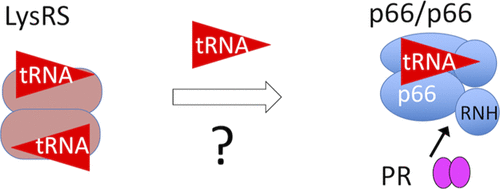
In human immunodeficiency virus-1 (HIV-1), reverse transcriptase (RT) is encoded as a 66 kDa protein, p66, in the Gag-Pol polyprotein. This protein is proteolytically cleaved by HIV-1 protease (PR) to finally generate a mature RT that is a heterodimer, composed of a p66 subunit and a p66-derived 51 kDa subunit, p51. In our prior work, we demonstrated that tRNALys3 binding to p66/p66 facilitates efficient cleavage of p66 to p51 by PR. However, tRNALys3 is known to be recruited to the virus by forming a complex with lysyl–tRNA synthetase (LysRS). Herein, we tested whether LysRS can have an effect on RT maturation in vitro. Importantly, our data show no significant differences in RT maturation in the presence of LysRS. Furthermore, no apparent p66/66 interaction with LysRS was observed. Although PR cleaved LysRS, it did not immediately release tRNALys3 from LysRS. Thus, we conclude that a free fraction of tRNALys3, which is in equilibrium with a LysRS-bound form, interacts with p66/p66 without any additional mechanism involving release of tRNALys3 from LysRS. Given that only transient tRNALys3–p66/p66 interaction is needed for efficient RT maturation, a small amount of free tRNA may be sufficient for this process. These studies reveal molecular level insights into RT maturation and will be useful for the design of cellular/viral experiments to better understand the role of tRNA in HIV-1 replication.
中文翻译:

赖氨酰-tRNA合成酶对HIV-1逆转录酶成熟的影响。
在人类免疫缺陷病毒1(HIV-1)中,逆转录酶(RT)在Gag-Pol多蛋白中编码为66 kDa蛋白p66。该蛋白被HIV-1蛋白酶(PR)进行蛋白水解切割,最终生成一个成熟的RT,该RT是一种异源二聚体,由p66亚基和p66衍生的51 kDa亚基p51组成。在我们先前的工作中,我们证明了tRNA Lys3与p66 / p66的结合有助于PR有效地将p66裂解为p51。然而,已知通过与赖氨酰-tRNA合成酶(LysRS)形成复合体,tRNA Lys3被募集到病毒中。在这里,我们测试了LysRS是否可以在体外对RT成熟产生影响。重要的是,我们的数据显示在存在LysRS的情况下,RT成熟度没有显着差异。此外,未观察到与LysRS的明显的p66 / 66相互作用。虽然PR裂解LysRS,它并没有立即释放的tRNA Lys3从LysRS。因此,我们得出结论,以与LysRS结合的形式处于平衡状态的tRNA Lys3的自由部分与p66 / p66相互作用,而没有涉及从LysRS释放tRNA Lys3的任何其他机制。鉴于只有短暂的tRNA Lys3–p66 / p66相互作用需要有效的RT成熟,少量的游离tRNA可能足以完成此过程。这些研究揭示了分子水平对RT成熟的见解,将有助于设计细胞/病毒实验,以更好地了解tRNA在HIV-1复制中的作用。
更新日期:2020-07-14
中文翻译:

赖氨酰-tRNA合成酶对HIV-1逆转录酶成熟的影响。
在人类免疫缺陷病毒1(HIV-1)中,逆转录酶(RT)在Gag-Pol多蛋白中编码为66 kDa蛋白p66。该蛋白被HIV-1蛋白酶(PR)进行蛋白水解切割,最终生成一个成熟的RT,该RT是一种异源二聚体,由p66亚基和p66衍生的51 kDa亚基p51组成。在我们先前的工作中,我们证明了tRNA Lys3与p66 / p66的结合有助于PR有效地将p66裂解为p51。然而,已知通过与赖氨酰-tRNA合成酶(LysRS)形成复合体,tRNA Lys3被募集到病毒中。在这里,我们测试了LysRS是否可以在体外对RT成熟产生影响。重要的是,我们的数据显示在存在LysRS的情况下,RT成熟度没有显着差异。此外,未观察到与LysRS的明显的p66 / 66相互作用。虽然PR裂解LysRS,它并没有立即释放的tRNA Lys3从LysRS。因此,我们得出结论,以与LysRS结合的形式处于平衡状态的tRNA Lys3的自由部分与p66 / p66相互作用,而没有涉及从LysRS释放tRNA Lys3的任何其他机制。鉴于只有短暂的tRNA Lys3–p66 / p66相互作用需要有效的RT成熟,少量的游离tRNA可能足以完成此过程。这些研究揭示了分子水平对RT成熟的见解,将有助于设计细胞/病毒实验,以更好地了解tRNA在HIV-1复制中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号