Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Reporting and Splitting Nanopomegranates Potentiate Deep Tissue Cancer Radiotherapy via Elevated Diffusion and Transcytosis.
ACS Nano ( IF 17.1 ) Pub Date : 2020-06-29 , DOI: 10.1021/acsnano.0c02674 Li Wang 1, 2 , Wei Jiang 1 , Liang Xiao 3 , Hongjun Li 2 , Ziqi Chen 2 , Yi Liu 2 , Jiaxiang Dou 2 , Shuya Li 2 , Qin Wang 2 , Wei Han 4 , Yucai Wang 1, 2 , Hang Liu 1, 2
ACS Nano ( IF 17.1 ) Pub Date : 2020-06-29 , DOI: 10.1021/acsnano.0c02674 Li Wang 1, 2 , Wei Jiang 1 , Liang Xiao 3 , Hongjun Li 2 , Ziqi Chen 2 , Yi Liu 2 , Jiaxiang Dou 2 , Shuya Li 2 , Qin Wang 2 , Wei Han 4 , Yucai Wang 1, 2 , Hang Liu 1, 2
Affiliation
|
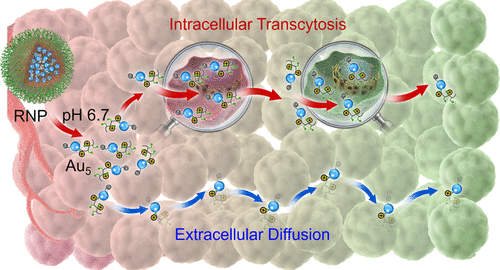
The efficacy of nanoradiosensitizers in cancer therapy has been primarily impeded by their limited accessibility to radioresistant cancer cells residing deep inside tumor tissues. The failure to report tumor response to radiotherapy generally delays adjustment of the treatment schedule and sets up another substantial obstacle to clinical success. Here, we develop a nanopomegranate (RNP) platform that not only visualizes the cancer radiosensitivities but also potentiates deep tissue cancer radiotherapy via elevated passive diffusion and active transcytosis. The RNPs are engineered through the programmed self-assembly of a tumor environment-targeting polymeric matrix and modular building blocks of ultrasmall gold nanoparticles (Au5). Once RNPs reach the tumors, the environmental acidity triggers the splitting and surface cationization of Au5. The small dimension of Au5 allows its passive diffusion, while positive surface charge enables its active transcytosis to cross the tumor interstitium. Meanwhile, the reporter element monitors the feedback of favorable radiotherapy responsiveness by detecting the activated apoptosis after radiation. The pivotal role of RNPs in improving and identifying radiotherapeutic outcomes is demonstrated in various tumor bearing mouse models with different radiosensitivities. In summary, our strategy offers a promising paradigm for deep tissue drug delivery as well as individualized precision radiotherapy.
中文翻译:

自我报告和分裂的纳米石榴可通过扩散和转胞作用增强深层组织癌的放射治疗。
纳米放射增敏剂在癌症治疗中的功效主要受到其对位于肿瘤组织深处的抗辐射癌细胞的有限访问的限制。未能报告对放疗的肿瘤反应通常会延迟治疗计划的调整,并为临床成功带来另一个重大障碍。在这里,我们开发了一个纳米石榴(RNP)平台,不仅可以可视化癌症的放射敏感性,还可以通过升高的被动扩散和主动转胞吞作用来增强深层组织的放射疗法。RNP通过靶向肿瘤环境的聚合物基体和超小金纳米颗粒的模块化构件的程序化自组装进行工程设计(Au 5)。一旦RNPs到达肿瘤,环境酸性会触发Au 5的分裂和表面阳离子化。小尺寸的Au 5允许其被动扩散,而正表面电荷使其主动转胞作用穿过肿瘤间质。同时,报道分子通过检测放射后激活的细胞凋亡来监测良好的放疗反应性反馈。在具有不同放射敏感性的各种荷瘤小鼠模型中证明了RNP在改善和鉴定放射治疗结果中的关键作用。总而言之,我们的策略为深层组织药物输送以及个性化精确放射治疗提供了有希望的范例。
更新日期:2020-07-28
中文翻译:

自我报告和分裂的纳米石榴可通过扩散和转胞作用增强深层组织癌的放射治疗。
纳米放射增敏剂在癌症治疗中的功效主要受到其对位于肿瘤组织深处的抗辐射癌细胞的有限访问的限制。未能报告对放疗的肿瘤反应通常会延迟治疗计划的调整,并为临床成功带来另一个重大障碍。在这里,我们开发了一个纳米石榴(RNP)平台,不仅可以可视化癌症的放射敏感性,还可以通过升高的被动扩散和主动转胞吞作用来增强深层组织的放射疗法。RNP通过靶向肿瘤环境的聚合物基体和超小金纳米颗粒的模块化构件的程序化自组装进行工程设计(Au 5)。一旦RNPs到达肿瘤,环境酸性会触发Au 5的分裂和表面阳离子化。小尺寸的Au 5允许其被动扩散,而正表面电荷使其主动转胞作用穿过肿瘤间质。同时,报道分子通过检测放射后激活的细胞凋亡来监测良好的放疗反应性反馈。在具有不同放射敏感性的各种荷瘤小鼠模型中证明了RNP在改善和鉴定放射治疗结果中的关键作用。总而言之,我们的策略为深层组织药物输送以及个性化精确放射治疗提供了有希望的范例。


























 京公网安备 11010802027423号
京公网安备 11010802027423号