Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functional and structural characterization of PII‐like protein CutA does not support involvement in heavy metal tolerance and hints at a small‐molecule carrying/signaling role
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-06-29 , DOI: 10.1111/febs.15464 Khaled A Selim 1, 2 , Lorena Tremiño 3 , Clara Marco-Marín 3 , Vikram Alva 2 , Javier Espinosa 4 , Asunción Contreras 4 , Marcus D Hartmann 2 , Karl Forchhammer 1 , Vicente Rubio 3
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-06-29 , DOI: 10.1111/febs.15464 Khaled A Selim 1, 2 , Lorena Tremiño 3 , Clara Marco-Marín 3 , Vikram Alva 2 , Javier Espinosa 4 , Asunción Contreras 4 , Marcus D Hartmann 2 , Karl Forchhammer 1 , Vicente Rubio 3
Affiliation
|
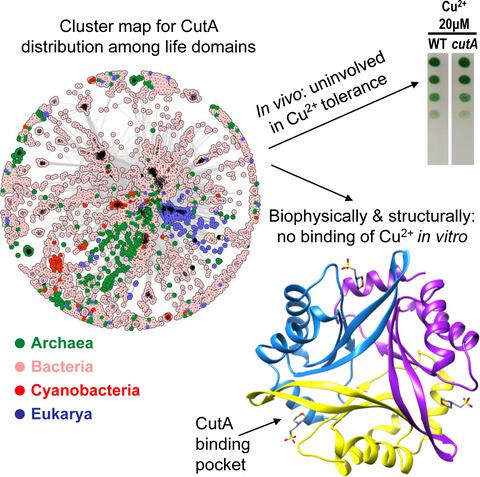
The PII‐like protein CutA is annotated as being involved in Cu2+ tolerance, based on analysis of Escherichia coli mutants. However, the precise cellular function of CutA remains unclear. Our bioinformatic analysis reveals that CutA proteins are universally distributed across all domains of life. Based on sequence‐based clustering, we chose representative cyanobacterial CutA proteins for physiological, biochemical, and structural characterization and examined their involvement in heavy metal tolerance, by generating CutA mutants in filamentous Nostoc sp. and in unicellular Synechococcus elongatus. However, we were unable to find any involvement of cyanobacterial CutA in metal tolerance under various conditions. This prompted us to re‐examine experimentally the role of CutA in protecting E. coli from Cu2+. Since we found no effect on copper tolerance, we conclude that CutA plays a different role that is not involved in metal protection. We resolved high‐resolution CutA structures from Nostoc and S. elongatus. Similarly to their counterpart from E. coli and to canonical PII proteins, cyanobacterial CutA proteins are trimeric in solution and in crystal structure; however, no binding affinity for small signaling molecules or for Cu2+ could be detected. The clefts between the CutA subunits, corresponding to the binding pockets of PII proteins, are formed by conserved aromatic and charged residues, suggesting a conserved binding/signaling function for CutA. In fact, we find binding of organic Bis‐Tris/MES molecules in CutA crystal structures, revealing a strong tendency of these pockets to accommodate cargo. This highlights the need to search for the potential physiological ligands and for their signaling functions upon binding to CutA.
中文翻译:

PII样蛋白CutA的功能和结构表征不支持参与重金属耐受性,并暗示小分子携带/信号传导作用
基于对大肠杆菌突变体的分析,PII类蛋白CutA被注释为参与Cu 2+耐受性。但是,CutA的确切细胞功能仍不清楚。我们的生物信息学分析表明,CutA蛋白普遍分布于生活的所有领域。基于基于序列的聚类,我们选择了代表性的蓝细菌CutA蛋白进行了生理,生化和结构表征,并通过在丝状Nostoc sp中生成CutA突变体来检查了它们对重金属耐受性的参与。和在单细胞中延伸。但是,我们无法发现在各种条件下蓝细菌CutA都对金属耐受性有任何影响。这促使我们在实验上重新审查CutA在保护大肠杆菌免受Cu 2+侵害中的作用。由于我们发现对铜的耐受性没有影响,因此可以得出结论,CutA扮演着与金属保护无关的角色。我们解析了Nostoc和S. elongatus的高分辨率CutA结构。与大肠杆菌和标准PII蛋白类似,蓝细菌CutA蛋白在溶液和晶体结构中都是三聚体。但是,对小信号分子或Cu 2+没有结合亲和力可以被检测到。CutA亚基之间的裂口,对应于PII蛋白的结合口袋,是由保守的芳香族和带电残基形成的,这暗示了CutA的保守结合/信号传导功能。实际上,我们在CutA晶体结构中发现了有机Bis-Tris / MES分子的结合,这表明这些口袋可容纳货物的强烈趋势。这突出显示了在与CutA结合后需要寻找潜在的生理配体及其信号传导功能的需求。
更新日期:2020-06-29
中文翻译:

PII样蛋白CutA的功能和结构表征不支持参与重金属耐受性,并暗示小分子携带/信号传导作用
基于对大肠杆菌突变体的分析,PII类蛋白CutA被注释为参与Cu 2+耐受性。但是,CutA的确切细胞功能仍不清楚。我们的生物信息学分析表明,CutA蛋白普遍分布于生活的所有领域。基于基于序列的聚类,我们选择了代表性的蓝细菌CutA蛋白进行了生理,生化和结构表征,并通过在丝状Nostoc sp中生成CutA突变体来检查了它们对重金属耐受性的参与。和在单细胞中延伸。但是,我们无法发现在各种条件下蓝细菌CutA都对金属耐受性有任何影响。这促使我们在实验上重新审查CutA在保护大肠杆菌免受Cu 2+侵害中的作用。由于我们发现对铜的耐受性没有影响,因此可以得出结论,CutA扮演着与金属保护无关的角色。我们解析了Nostoc和S. elongatus的高分辨率CutA结构。与大肠杆菌和标准PII蛋白类似,蓝细菌CutA蛋白在溶液和晶体结构中都是三聚体。但是,对小信号分子或Cu 2+没有结合亲和力可以被检测到。CutA亚基之间的裂口,对应于PII蛋白的结合口袋,是由保守的芳香族和带电残基形成的,这暗示了CutA的保守结合/信号传导功能。实际上,我们在CutA晶体结构中发现了有机Bis-Tris / MES分子的结合,这表明这些口袋可容纳货物的强烈趋势。这突出显示了在与CutA结合后需要寻找潜在的生理配体及其信号传导功能的需求。


























 京公网安备 11010802027423号
京公网安备 11010802027423号