Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The intrinsic ATPase activity of Mycobacterium tuberculosis UvrC is crucial for its damage‐specific DNA incision function
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-06-29 , DOI: 10.1111/febs.15465 Manoj Thakur 1 , Ankit Agarwal 1 , Kalappa Muniyappa 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-06-29 , DOI: 10.1111/febs.15465 Manoj Thakur 1 , Ankit Agarwal 1 , Kalappa Muniyappa 1
Affiliation
|
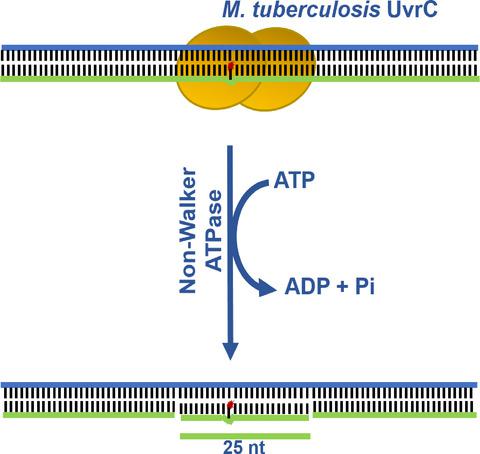
To ensure genome stability, bacteria have evolved a network of DNA repair mechanisms; among them, the UvrABC‐dependent nucleotide excision repair (NER) pathway is essential for the incision of a variety of bulky adducts generated by exogenous chemicals, UV radiation and by‐products of cellular metabolism. However, very little is known about the enzymatic properties of Mycobacterium tuberculosis UvrABC excinuclease complex. Furthermore, the biochemical properties of Escherichia coli UvrC (EcUvrC) are not well understood (compared to UvrA and UvrB), perhaps due to its limited availability and/or activity instability in vitro. In addition, homology modelling of M. tuberculosis UvrC (MtUvrC) revealed the presence of a putative ATP‐binding pocket, although its function remains unknown. To elucidate the biochemical properties of UvrC, we constructed and purified wild‐type MtUvrC and its eight variants harbouring mutations within the ATP‐binding pocket. The data from DNA‐binding studies suggest that MtUvrC exhibits high‐affinity for duplex DNA containing a bubble or fluorescein‐dT moiety, over fluorescein‐adducted single‐stranded DNA. Most notably, MtUvrC has an intrinsic UvrB‐independent ATPase activity, which drives dual incision of the damaged DNA strand. In contrast, EcUvrC is devoid of ATPase activity; however, it retains the ability to bind ATP at levels comparable to that of MtUvrC. The ATPase‐deficient variants map to residues lining the MtUvrC ATP‐binding pocket. Further analysis of these variants revealed separation of function between ATPase and DNA‐binding activities in MtUvrC. Altogether, these findings reveal functional diversity of the bacterial NER machinery and a paradigm for the evolution of a catalytic scaffold in UvrC.
中文翻译:

结核分枝杆菌UvrC的固有ATPase活性对于其损伤特异性DNA切口功能至关重要
为了确保基因组的稳定性,细菌已经进化出DNA修复机制的网络。其中,UvrABC依赖的核苷酸切除修复(NER)途径对于切割由外源化学物质,紫外线辐射和细胞代谢副产物产生的各种大体积加合物至关重要。但是,关于结核分枝杆菌UvrABC核酸外切酶复合物的酶学性质知之甚少。此外,大肠杆菌UvrC(EcUvrC)的生化特性尚未得到很好的理解(与UvrA和UvrB相比),这可能是由于其有限的可用性和/或体外的活性不稳定。此外,结核分枝杆菌的同源性建模UvrC(MtUvrC)揭示了假定的ATP结合口袋的存在,尽管其功能仍然未知。为了阐明UvrC的生化特性,我们构建并纯化了野生型MtUvrC及其8个在ATP结合口袋中带有突变的变体。DNA结合研究的数据表明,与荧光素加成的单链DNA相比,MtUvrC对包含气泡或荧光素dT部分的双链DNA具有高亲和力。最值得注意的是,MtUvrC具有固有的UvrB独立ATPase活性,可驱动受损DNA链的双重切割。相反,EcUvrC没有ATPase活性。但是,它保留了以与MtUvrC相当的水平结合ATP的能力。ATPase缺失的变体映射到MtUvrC ATP结合袋内衬的残基。对这些变体的进一步分析揭示了MtUvrC中ATPase和DNA结合活性之间的功能分离。总而言之,这些发现揭示了细菌NER机器的功能多样性以及UvrC中催化支架进化的范例。
更新日期:2020-06-29
中文翻译:

结核分枝杆菌UvrC的固有ATPase活性对于其损伤特异性DNA切口功能至关重要
为了确保基因组的稳定性,细菌已经进化出DNA修复机制的网络。其中,UvrABC依赖的核苷酸切除修复(NER)途径对于切割由外源化学物质,紫外线辐射和细胞代谢副产物产生的各种大体积加合物至关重要。但是,关于结核分枝杆菌UvrABC核酸外切酶复合物的酶学性质知之甚少。此外,大肠杆菌UvrC(EcUvrC)的生化特性尚未得到很好的理解(与UvrA和UvrB相比),这可能是由于其有限的可用性和/或体外的活性不稳定。此外,结核分枝杆菌的同源性建模UvrC(MtUvrC)揭示了假定的ATP结合口袋的存在,尽管其功能仍然未知。为了阐明UvrC的生化特性,我们构建并纯化了野生型MtUvrC及其8个在ATP结合口袋中带有突变的变体。DNA结合研究的数据表明,与荧光素加成的单链DNA相比,MtUvrC对包含气泡或荧光素dT部分的双链DNA具有高亲和力。最值得注意的是,MtUvrC具有固有的UvrB独立ATPase活性,可驱动受损DNA链的双重切割。相反,EcUvrC没有ATPase活性。但是,它保留了以与MtUvrC相当的水平结合ATP的能力。ATPase缺失的变体映射到MtUvrC ATP结合袋内衬的残基。对这些变体的进一步分析揭示了MtUvrC中ATPase和DNA结合活性之间的功能分离。总而言之,这些发现揭示了细菌NER机器的功能多样性以及UvrC中催化支架进化的范例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号