当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structures of two isostructural compounds: a second polymorph of dipotassium hydrogen citrate, K2HC6H5O7, and potassium rubidium hydrogen citrate, KRbHC6H5O7.
Acta Crystallographica Section C ( IF 0.8 ) Pub Date : 2020-06-30 , DOI: 10.1107/s2053229620008281 Diana Gonzalez 1 , Joseph T Golab 1 , Andrew J Cigler 2 , James A Kaduk 2
Acta Crystallographica Section C ( IF 0.8 ) Pub Date : 2020-06-30 , DOI: 10.1107/s2053229620008281 Diana Gonzalez 1 , Joseph T Golab 1 , Andrew J Cigler 2 , James A Kaduk 2
Affiliation
|
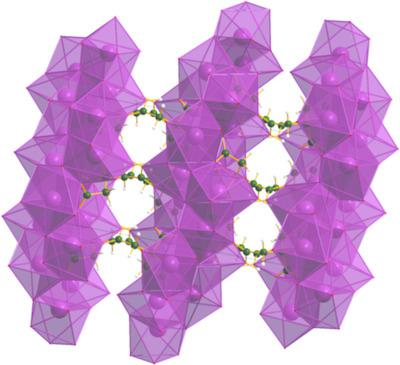
The crystal structures of a new polymorph of dipotassium hydrogen citrate, 2K+·HC6H5O72−, and potassium rubidium hydrogen citrate, K+·Rb+·HC6H5O72−, have been solved and refined using laboratory powder X‐ray diffraction and optimized using density functional techniques. In the new polymorph of the dipotassium salt, KO7 and KO8 coordination polyhedra share corners and edges to form a three‐dimensional framework with channels parallel to the a axis and [111]. The hydrophobic methylene groups face each other in the channels. The un‐ionized carboxylic acid group forms a strong charge‐assisted hydrogen bond to the central ionized carboxylate group. The hydroxy group forms an intermolecular hydrogen bond to a different central carboxylate group. In the potassium rubidium salt, the K+ and Rb+ cations are disordered over two sites, in approximately 0.72:0.28 and 0.28:0.72 ratios. KO8 and RbO9 coordination polyhedra share corners and edges to form a three‐dimensional framework with channels parallel to the a axis. The un‐ionized carboxylic acid group forms a strong charge‐assisted hydrogen bond to an ionized carboxylate group. The hydroxy group forms an intermolecular hydrogen bond to the central carboxylate group. Density functional theory (DFT) calculations on the ordered cation structures suggest that interchange of K+ and Rb+ at the two cation sites changes the energy insignificantly.
中文翻译:

两种同构化合物的晶体结构:柠檬酸氢二钾的第二个多晶型物K2HC6H5O7和柠檬酸氢钾rub的KRbHC6H5O7。
解决并精制了一种新的柠檬酸氢二钾2K + ·HC 6 H 5 O 7 2-的多晶型物和柠檬酸氢钾potassium K + ·Rb + ·HC 6 H 5 O 7 2-的晶体结构。使用实验室粉末X射线衍射,并使用密度泛函技术进行优化。在新的双钾盐多晶型物中,KO 7和KO 8配位多面体共享角和边,以形成三维框架,其通道平行于α轴和[111]。疏水亚甲基在通道中彼此面对。未电离的羧酸基团与中心电离的羧酸盐基团形成一个强大的电荷辅助氢键。羟基与不同的中心羧酸酯基形成分子间氢键。在钾rub盐中,K +和Rb +阳离子在两个位点上以大约0.72:0.28和0.28:0.72的比率无序排列。KO 8和RbO 9配位多面体共享角和边,以形成三维框架,其通道平行于a轴。未电离的羧酸基团与电离的羧酸酯基团形成强电荷辅助氢键。羟基与中心羧酸酯基形成分子间氢键。对有序阳离子结构的密度泛函理论(DFT)计算表明,两个阳离子位点处的K +和Rb +互换不会显着改变能量。
更新日期:2020-06-30
中文翻译:

两种同构化合物的晶体结构:柠檬酸氢二钾的第二个多晶型物K2HC6H5O7和柠檬酸氢钾rub的KRbHC6H5O7。
解决并精制了一种新的柠檬酸氢二钾2K + ·HC 6 H 5 O 7 2-的多晶型物和柠檬酸氢钾potassium K + ·Rb + ·HC 6 H 5 O 7 2-的晶体结构。使用实验室粉末X射线衍射,并使用密度泛函技术进行优化。在新的双钾盐多晶型物中,KO 7和KO 8配位多面体共享角和边,以形成三维框架,其通道平行于α轴和[111]。疏水亚甲基在通道中彼此面对。未电离的羧酸基团与中心电离的羧酸盐基团形成一个强大的电荷辅助氢键。羟基与不同的中心羧酸酯基形成分子间氢键。在钾rub盐中,K +和Rb +阳离子在两个位点上以大约0.72:0.28和0.28:0.72的比率无序排列。KO 8和RbO 9配位多面体共享角和边,以形成三维框架,其通道平行于a轴。未电离的羧酸基团与电离的羧酸酯基团形成强电荷辅助氢键。羟基与中心羧酸酯基形成分子间氢键。对有序阳离子结构的密度泛函理论(DFT)计算表明,两个阳离子位点处的K +和Rb +互换不会显着改变能量。


























 京公网安备 11010802027423号
京公网安备 11010802027423号