当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coinhibition of activated p38 MAPKα and mTORC1 potentiates stemness maintenance of HSCs from SR1-expanded human cord blood CD34+ cells via inhibition of senescence.
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-06-29 , DOI: 10.1002/sctm.20-0129 Xiaoyi Li 1 , Xiao Ma 2 , Ying Chen 2 , Danyue Peng 2 , Huifang Wang 2 , Suhua Chen 3 , Yin Xiao 4 , Lei Li 5 , Hao Zhou 2 , Fanjun Cheng 2 , Yingdai Gao 6 , Jiwei Chang 7 , Tao Cheng 6 , Lingbo Liu 2
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-06-29 , DOI: 10.1002/sctm.20-0129 Xiaoyi Li 1 , Xiao Ma 2 , Ying Chen 2 , Danyue Peng 2 , Huifang Wang 2 , Suhua Chen 3 , Yin Xiao 4 , Lei Li 5 , Hao Zhou 2 , Fanjun Cheng 2 , Yingdai Gao 6 , Jiwei Chang 7 , Tao Cheng 6 , Lingbo Liu 2
Affiliation
|
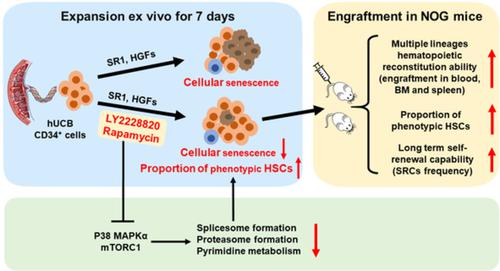
The stemness of ex vivo expanded hematopoietic stem cells (HSCs) is usually compromised by current methods. To explore the failure mechanism of stemness maintenance of human HSCs, which were expanded from human umbilical cord blood (hUCB) CD34+ cells, by differentiation inhibitor Stem Regenin 1 (SR1), an antagonist of aryl hydrocarbon receptor, we investigated the activity of p38 mitogen‐activated protein kinase α (p38 MAPKα, p38α) and mammalian target of rapamycin complex 1 (mTORC1), and their effect on SR1‐expanded hUCB CD34+ cells. Our results showed that cellular senescence occurred in the SR1‐expanded hUCB CD34+ cells in which p38α and mTORC1 were successively activated. Furthermore, their coinhibition resulted in a further decrease in hUCB CD34+ cell senescence without an effect on apoptosis, promoted the maintenance of expanded phenotypic HSCs without differentiation inhibition, increased the hematopoietic reconstitution ability of multiple lineages, and potentiated the long‐term self‐renewal capability of HSCs from SR1‐expanded hUCB CD34+ cells in NOD/Shi‐scid/IL‐2Rγnull mice. Our mechanistic study revealed that senescence inhibition by our strategy was mainly attributed to downregulation of the splicesome, proteasome formation, and pyrimidine metabolism signaling pathways. These results suggest that coinhibition of activated p38α and mTORC1 potentiates stemness maintenance of HSCs from SR1‐expanded hUCB CD34+ cells via senescence inhibition. Thus, we established a new strategy to maintain the stemness of ex vivo differentiation inhibitor‐expanded human HSCs via coinhibition of multiple independent senescence initiating signal pathways. This senescence inhibition‐induced stemness maintenance of ex vivo expanded HSCs could also have an important role in other HSC expansion systems.
中文翻译:

激活的 p38 MAPKα 和 mTORC1 的共同抑制通过抑制衰老增强了来自 SR1 扩增的人脐带血 CD34+ 细胞的 HSC 的干细胞维持。
体外扩增造血干细胞 (HSC) 的干性通常会受到当前方法的影响。为了探索从人脐带血 (hUCB) CD34 +细胞扩增而来的人 HSC 的干细胞维持失败机制,通过分化抑制剂 Stem Regenin 1 (SR1),一种芳烃受体的拮抗剂,我们研究了 p38 的活性丝裂原活化蛋白激酶α(p38 MAPKα、p38α)和哺乳动物雷帕霉素复合物1(mTORC1)靶点,以及它们对SR1扩增的hUCB CD34 +细胞的影响。我们的结果表明,细胞衰老发生在 SR1 扩增的 hUCB CD34 +细胞中,其中 p38α 和 mTORC1 相继被激活。此外,它们的共抑制导致 hUCB CD34+细胞衰老对细胞凋亡没有影响,促进扩增表型 HSC 的维持而不抑制分化,增加多个谱系的造血重建能力,并增强来自 SR1 扩增的 hUCB CD34 +细胞的 HSC 的长期自我更新能力NOD/Shi-scid/IL-2Rγ缺失小鼠。我们的机制研究表明,我们的策略对衰老的抑制主要归因于剪接体、蛋白酶体形成和嘧啶代谢信号通路的下调。这些结果表明激活的 p38α 和 mTORC1 的共同抑制增强了来自 SR1 扩增的 hUCB CD34 +的 HSC 的干性维持细胞通过衰老抑制。因此,我们建立了一种新策略,通过共同抑制多个独立的衰老启动信号通路来维持体外分化抑制剂扩增的人类 HSC 的干性。体外扩增的 HSC 的这种衰老抑制诱导的干细胞维持也可能在其他 HSC 扩增系统中发挥重要作用。
更新日期:2020-06-29
中文翻译:

激活的 p38 MAPKα 和 mTORC1 的共同抑制通过抑制衰老增强了来自 SR1 扩增的人脐带血 CD34+ 细胞的 HSC 的干细胞维持。
体外扩增造血干细胞 (HSC) 的干性通常会受到当前方法的影响。为了探索从人脐带血 (hUCB) CD34 +细胞扩增而来的人 HSC 的干细胞维持失败机制,通过分化抑制剂 Stem Regenin 1 (SR1),一种芳烃受体的拮抗剂,我们研究了 p38 的活性丝裂原活化蛋白激酶α(p38 MAPKα、p38α)和哺乳动物雷帕霉素复合物1(mTORC1)靶点,以及它们对SR1扩增的hUCB CD34 +细胞的影响。我们的结果表明,细胞衰老发生在 SR1 扩增的 hUCB CD34 +细胞中,其中 p38α 和 mTORC1 相继被激活。此外,它们的共抑制导致 hUCB CD34+细胞衰老对细胞凋亡没有影响,促进扩增表型 HSC 的维持而不抑制分化,增加多个谱系的造血重建能力,并增强来自 SR1 扩增的 hUCB CD34 +细胞的 HSC 的长期自我更新能力NOD/Shi-scid/IL-2Rγ缺失小鼠。我们的机制研究表明,我们的策略对衰老的抑制主要归因于剪接体、蛋白酶体形成和嘧啶代谢信号通路的下调。这些结果表明激活的 p38α 和 mTORC1 的共同抑制增强了来自 SR1 扩增的 hUCB CD34 +的 HSC 的干性维持细胞通过衰老抑制。因此,我们建立了一种新策略,通过共同抑制多个独立的衰老启动信号通路来维持体外分化抑制剂扩增的人类 HSC 的干性。体外扩增的 HSC 的这种衰老抑制诱导的干细胞维持也可能在其他 HSC 扩增系统中发挥重要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号