Materials Today Communications ( IF 3.8 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.mtcomm.2020.101382 Alireza Cheraghi , Hanka Becker , Hosna Eftekhari , Hossein Yoozbashizadeh , Jafar Safarian
|
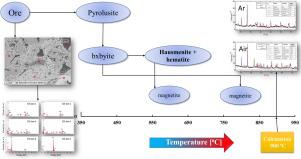
Characterization and calcination behavior of a low-grade manganese ore, as a part of Mn ferroalloys production, was studied by XRF, ex-situ XRD, in-situ XRD, and SEM-EDS techniques. Calcination experiments were carried out at and up to 900 °C (1173 K) in air and argon atmospheres. The samples were in particles and powder forms. The results indicated that both quartz and calcite phases in the ore exhibit a bimodal spatial distribution; as relatively large regions and finely distributed in the Mn- and Fe-containing phases. By Rietveld analysis of the in-situ XRD data, the reactions occurring upon heating during the calcination process were deduced. Thermal decomposition and reactive diffusion were found to be the dominant mechanisms in the calcination process. The results demonstrated that there were some phases forming and vanishing during the calcination process in air, such as calcium ferrites, calcium oxide, and bixbyite. While Fe-containing bixbyite was thermally decomposed to hausmannite at low temperatures, magnetite was the product at higher temperatures. However, magnetite was also formed through the diffusion between hematite and hausmannite phases. Reactive diffusion was much more prevalent in fine ore powder form than particles. Formation of complex phases such as (di-)calcium ferrite and bustamite was the result of the reactive diffusion mechanism. Although thermal decomposition of Mn and Fe oxides was not influenced by particle size, the final product of calcination was highly affected by the particle size, as a result of reactive diffusion.
中文翻译:

低品位锰矿的表征与煅烧行为
通过XRF,异位XRD,原位XRD和SEM-EDS技术研究了作为锰铁合金生产一部分的低品位锰矿的表征和煅烧行为。煅烧实验是在空气和氩气气氛中,在最高900°C(1173 K)的温度下进行的。样品为颗粒和粉末形式。结果表明,矿石中的石英相和方解石相均表现出双峰空间分布。分布在相对较大的区域,并精细分布在含锰和铁相中。通过Rietveld对原位XRD数据的分析,可以推断出煅烧过程中加热时发生的反应。发现热分解和反应扩散是煅烧过程中的主要机理。结果表明,在空气中煅烧过程中有一些相形成和消失,例如铁酸钙,氧化钙和方钠石。含铁的方铁锰矿在低温下会热分解成菱锰矿,而磁铁矿是高温下的产物。但是,磁铁矿也是通过赤铁矿和苏曼石相之间的扩散形成的。在矿粉中,反应性扩散比颗粒性更为普遍。复杂相的形成,例如(二)铁酸钙和白铁矿是反应扩散机制的结果。尽管Mn和Fe氧化物的热分解不受粒度影响,但是由于反应扩散,煅烧的最终产物受粒度极大影响。氧化钙和方钠石。含铁的方铁锰矿在低温下会热分解成菱锰矿,而磁铁矿是高温下的产物。但是,磁铁矿也是通过赤铁矿和苏曼石相之间的扩散形成的。在矿粉中,反应性扩散比颗粒性更为普遍。诸如(二)钙铁氧体和白铁矿的复杂相的形成是反应扩散机理的结果。尽管Mn和Fe氧化物的热分解不受粒度的影响,但是由于反应扩散,煅烧的最终产物受粒度的影响很大。氧化钙和方钠石。含铁的方铁锰矿在低温下会热分解成菱锰矿,而磁铁矿是高温下的产物。但是,磁铁矿也是通过赤铁矿和苏曼石相之间的扩散形成的。在矿粉中,反应性扩散比颗粒性更为普遍。复杂相的形成,例如(二)铁酸钙和白铁矿是反应扩散机制的结果。尽管Mn和Fe氧化物的热分解不受粒度的影响,但是由于反应扩散,煅烧的最终产物受粒度的影响很大。磁铁矿也是通过赤铁矿和钙锰矿相之间的扩散形成的。在矿粉中,反应性扩散比颗粒性更为普遍。复杂相的形成,例如(二)铁酸钙和白铁矿是反应扩散机制的结果。尽管Mn和Fe氧化物的热分解不受粒度的影响,但是由于反应扩散,煅烧的最终产物受粒度的影响很大。磁铁矿也是通过赤铁矿和钙锰矿相之间的扩散形成的。在矿粉中,反应性扩散比颗粒性更为普遍。复杂相的形成,例如(二)铁酸钙和白铁矿是反应扩散机制的结果。尽管Mn和Fe氧化物的热分解不受粒度的影响,但是由于反应扩散,煅烧的最终产物受粒度的影响很大。


























 京公网安备 11010802027423号
京公网安备 11010802027423号