Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.3 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.jphotochem.2020.112738 Matthew G Romei 1 , Chi-Yun Lin 1 , Steven G Boxer 1
|
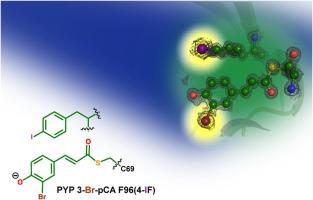
Photo-induced structural rearrangements of chromophore-containing proteins are essential for various light-dependent signaling pathways and optogenetic applications. Ultrafast structural and spectroscopic methods have offered insights into these structural rearrangements across many timescales. However, questions still remain about exact mechanistic details, especially regarding photoisomerization of the chromophore within these proteins femtoseconds to picoseconds after photoexcitation. Instrumentation advancements for time-resolved crystallography and ultrafast electron diffraction provide a promising opportunity to study these reactions, but achieving enough signal-to-noise is a constant challenge. Here we present four new photoactive yellow protein constructs and one new fluorescent protein construct that contain heavy atoms either within or around the chromophore and can be expressed with high yields. Structural characterization of these constructs, most at atomic resolution, show minimal perturbation caused by the heavy atoms compared to wild-type structures. Spectroscopic studies report the effects of the heavy atom identity and location on the chromophore’s photophysical properties. None of the substitutions prevent photoisomerization, although certain rates within the photocycle may be affected. Overall, these new proteins containing heavy atoms are ideal samples for state-of-the-art time-resolved crystallography and electron diffraction experiments to elucidate crucial mechanistic information of photoisomerization.
中文翻译:

光活性黄色蛋白和含有重原子的光开关荧光蛋白结构的结构和光谱表征。
含发色团蛋白质的光诱导结构重排对于各种光依赖性信号通路和光遗传学应用至关重要。超快结构和光谱方法为跨多个时间尺度的这些结构重排提供了见解。然而,关于确切的机械细节的问题仍然存在,特别是关于这些蛋白质内发色团在光激发后飞秒到皮秒内的光异构化。时间分辨晶体学和超快电子衍射仪器的进步为研究这些反应提供了一个有希望的机会,但实现足够的信噪比是一个持续的挑战。在这里,我们展示了四种新的光活性黄色蛋白构建体和一种新的荧光蛋白构建体,它们在生色团内部或周围含有重原子,并且可以高产量表达。与野生型结构相比,这些构造的结构表征(大多数在原子分辨率下)显示由重原子引起的扰动最小。光谱研究报告了重原子身份和位置对发色团光物理特性的影响。尽管光循环中的某些速率可能会受到影响,但没有一种替代能阻止光异构化。总的来说,这些含有重原子的新蛋白质是最先进的时间分辨晶体学和电子衍射实验的理想样品,可用于阐明光致异构化的关键机制信息。


























 京公网安备 11010802027423号
京公网安备 11010802027423号