Cell Reports ( IF 8.8 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.celrep.2020.107837 Carlos M Guardia 1 , Xiao-Feng Tan 2 , Tengfei Lian 2 , Mitra S Rana 3 , Wenchang Zhou 4 , Eric T Christenson 3 , Augustus J Lowry 3 , José D Faraldo-Gómez 4 , Juan S Bonifacino 1 , Jiansen Jiang 2 , Anirban Banerjee 3
|
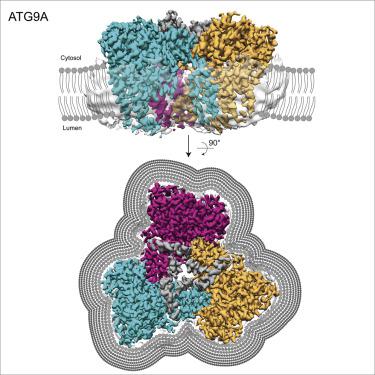
Autophagy is a catabolic process involving capture of cytoplasmic materials into double-membraned autophagosomes that subsequently fuse with lysosomes for degradation of the materials by lysosomal hydrolases. One of the least understood components of the autophagy machinery is the transmembrane protein ATG9. Here, we report a cryoelectron microscopy structure of the human ATG9A isoform at 2.9-Å resolution. The structure reveals a fold with a homotrimeric domain-swapped architecture, multiple membrane spans, and a network of branched cavities, consistent with ATG9A being a membrane transporter. Mutational analyses support a role for the cavities in the function of ATG9A. In addition, structure-guided molecular simulations predict that ATG9A causes membrane bending, explaining the localization of this protein to small vesicles and highly curved edges of growing autophagosomes.
中文翻译:

人ATG9A的结构,它是核心自噬机器的唯一跨膜蛋白。
自噬是一种分解代谢过程,涉及将细胞质材料捕获到双膜自噬体中,然后将其与溶酶体融合,通过溶酶体水解酶降解材料。自噬机制最不了解的组件之一是跨膜蛋白ATG9。在这里,我们报告人ATG9A亚型在2.9-Å分辨率下的低温电子显微镜结构。该结构显示出具有同源三聚体结构域交换结构的折叠,多个膜跨度和分支腔网络,与ATG9A是膜转运蛋白一致。突变分析支持空洞在ATG9A功能中的作用。此外,结构导向的分子模拟预测ATG9A会引起膜弯曲,


























 京公网安备 11010802027423号
京公网安备 11010802027423号